Production process of tricyclazole
A production process and technology for tricyclazole, applied in the field of production technology of tricyclazole, can solve problems such as inability to directly apply mechanically, environmental pollution, difficult purification, etc., reduce the time for distilling formic acid, reduce production cost, and achieve good refining effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
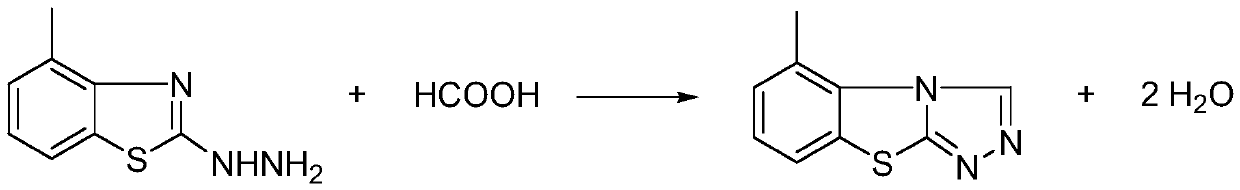
[0030] Raw materials: 4-methyl-2-hydrazinobenzothiazole (pesticide synthesis intermediate) 250kg
[0031] Cycling agent: 35% formic acid 1260kg
[0032] The first choice is to put 35% formic acid (1260kg) into 4-methyl-2-hydrazinobenzothiazole (250kg), heat up to 106°C for 3h under reflux, and carry out the reaction;
[0033] Secondly, after the reflux is completed, the temperature is raised to 153°C, and vacuum distillation is carried out to recover formic acid, and then water is added dropwise to the residue of vacuum distillation at a rate of 30 drops / min, cooled to 20°C for crystallization, filtered, and dried at 80°C Get the original drug of tricyclazole. The tricyclazole content in the obtained tricyclazole crude product is: 96%.
[0034] The crude product tricyclazole obtained is refined with ethyl acetate-ethanol mixed solvent (the volume ratio of ethyl acetate and ethanol is 2:1), and after the crude product tricyclazole is dissolved with ethyl acetate-ethanol mixed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com