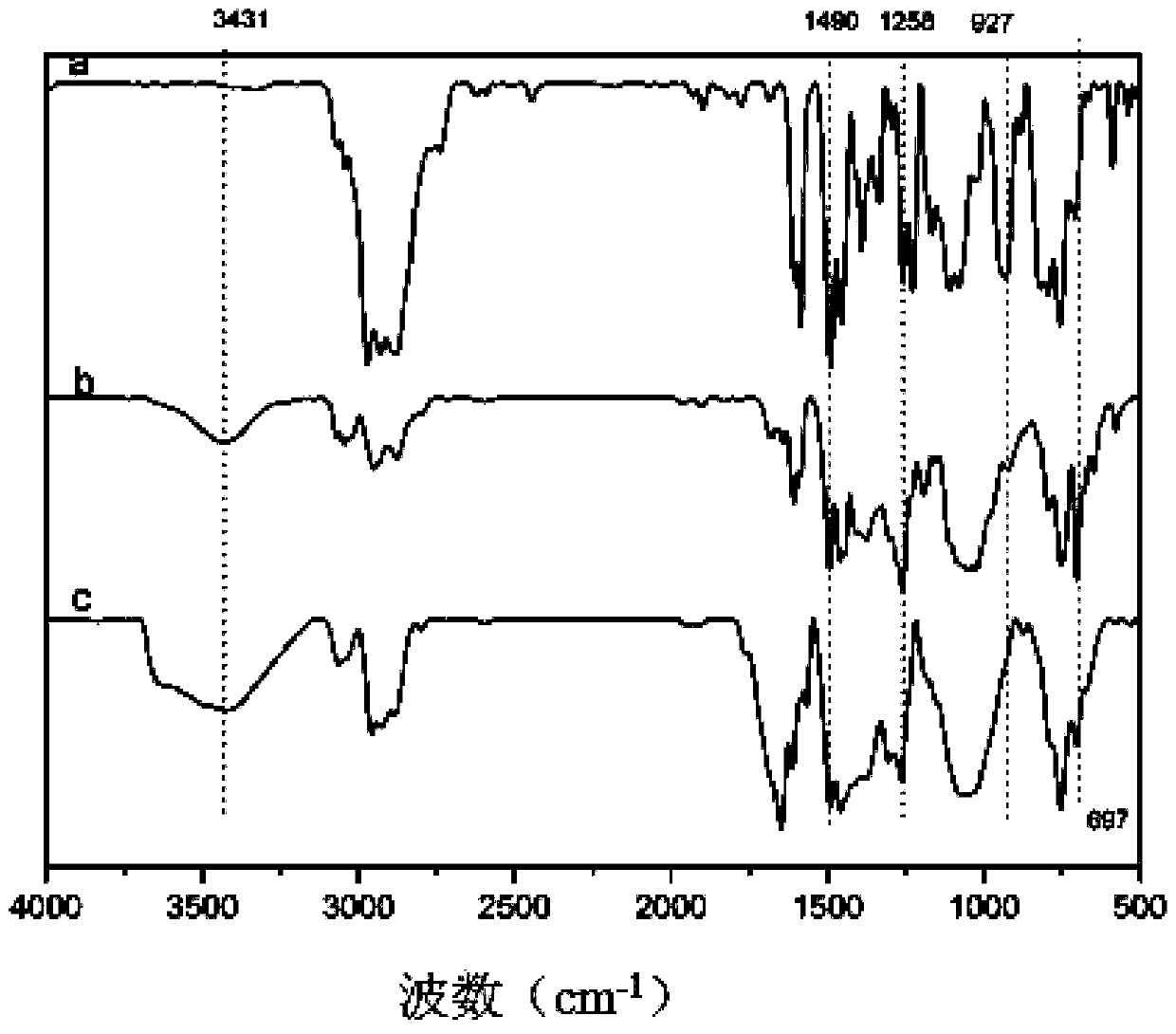
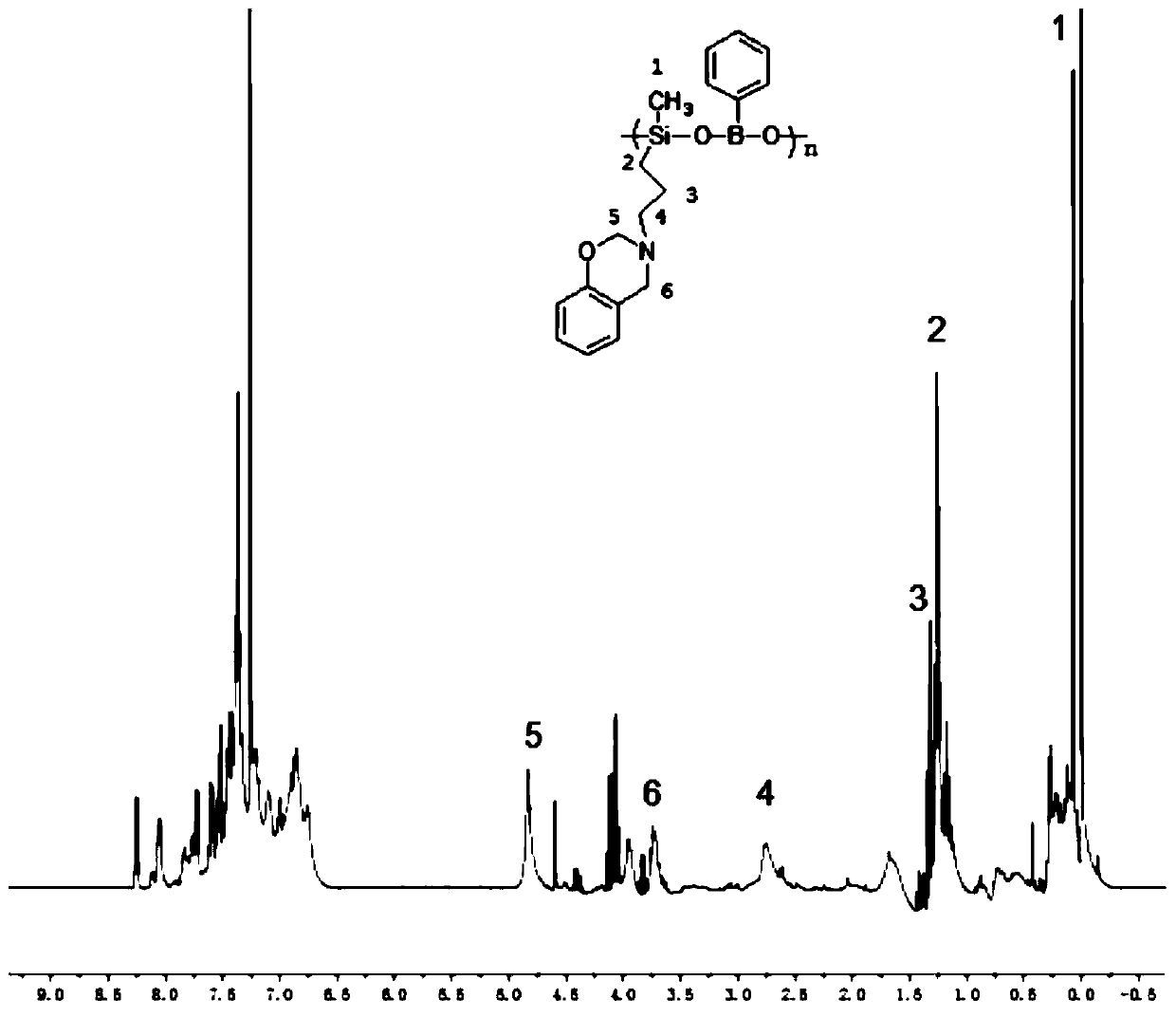
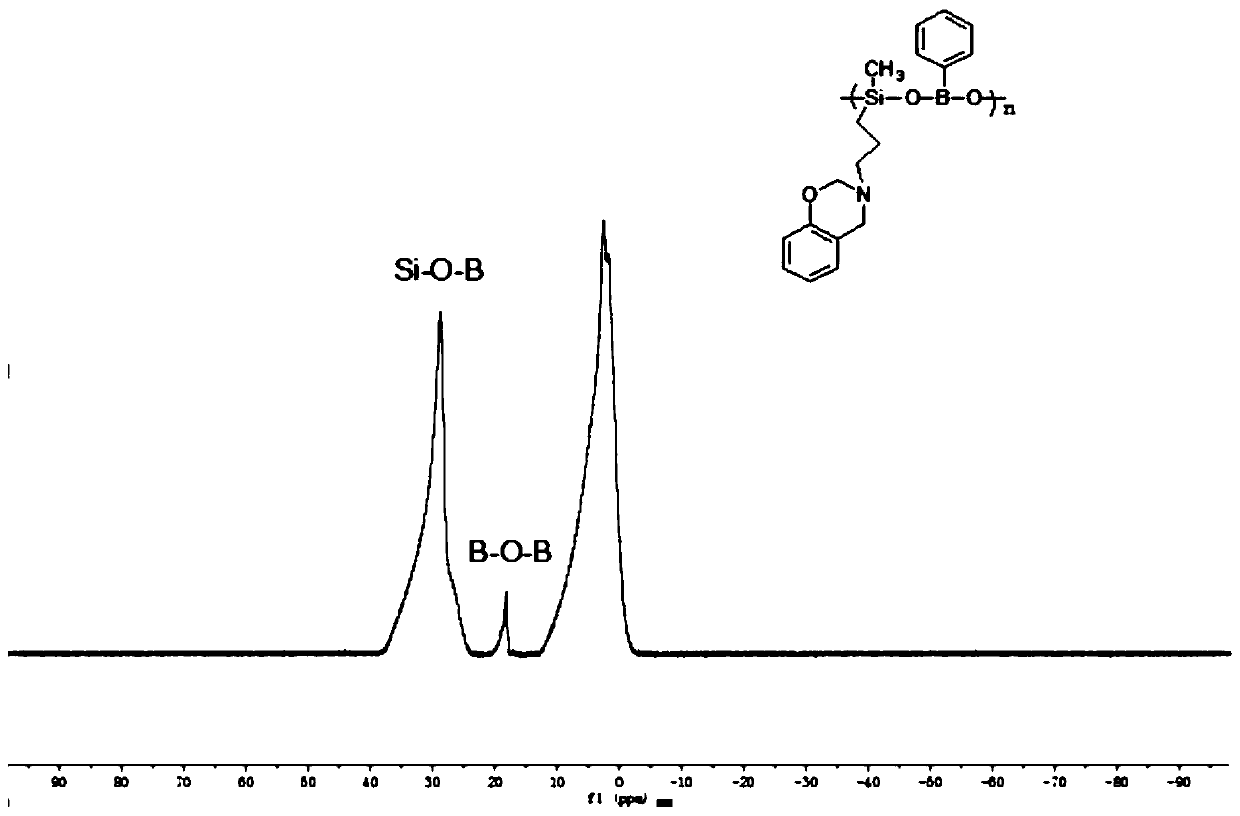
Polybenzoxazine cross-linked hydrolysis-resistant polyborosiloxane and preparation method thereof
A technology of polybenzoxazine and polyborosiloxane, which is applied in the field of new polyborosiloxane and its preparation, can solve problems such as poor hydrolysis resistance, achieve excellent hydrolysis resistance, simple and easy synthetic route, The effect of high Tg
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1. Synthesis of silicon-containing benzoxazine monomers based on phenol and γ-aminopropylmethyldimethoxysilane
[0070] Dissolve 0.25g of paraformaldehyde and 0.75g of γ-aminopropylmethyldimethoxysilane in 20mL of dioxane, stir the reaction at room temperature for 0.5h, add 0.44g of phenol, raise the temperature to 90°C, and react for 24h . Post-treatment: the solvent was removed by rotary evaporation, and the crude product was vacuum-dried at 60° C. to obtain a yellow liquid with a yield of 80%.
Embodiment 2
[0071] Example 2. Synthesis of silicon-containing benzoxazine monomers based on phenol and γ-aminopropylmethyldiethoxysilane
[0072] Dissolve 0.25g of paraformaldehyde and 0.51g of γ-aminopropylmethyldiethoxysilane in 20mL of dioxane, stir and mix at room temperature for 0.5h, add 0.44g of phenol, raise the temperature to 80°C, and react for 10h . Post-processing: the solvent was removed by rotary evaporation, and the crude product was vacuum-dried at 60° C. to obtain a yellow liquid with a yield of 62%.
Embodiment 3
[0073] Example 3, Synthesis of silicon-containing benzoxazine monomers based on bisphenol A and γ-aminopropylmethyldiethoxysilane
[0074] Dissolve 0.25g of paraformaldehyde and 0.51g of γ-aminopropylmethyldiethoxysilane in 20mL of dioxane, stir and mix at room temperature for 0.5h, add 0.64g of bisphenol A, and raise the temperature to 80°C. Reaction 10h. Post-processing: the solvent was removed by rotary evaporation, and the crude product was vacuum-dried at 60° C. to obtain a yellow liquid with a yield of 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com