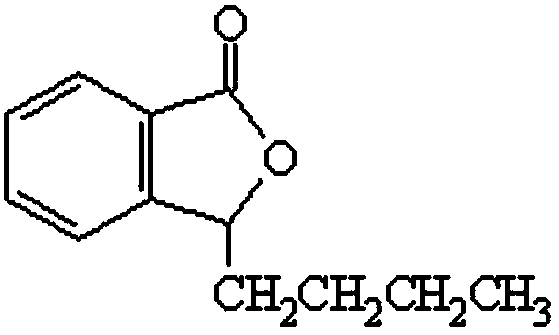
Stable 3-n-butylphthalide3-n-butylphthalide high-capacity injection and preparation method thereof
A technology of injection and butylphthalide, which is applied in the field of large-capacity injection of butylphthalide and its preparation, can solve problems such as product quality impact, difficult operation of dilution, and difficulty in clinical application, so as to make up for market gaps and avoid safety risks Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, large-volume injection of butylphthalide, comprising racemic butylphthalide or L-butylphthalide, polyethylene glycol-15-hydroxystearate, sodium chloride and water for injection.
[0051] Prescription 1, calculated in 1000ml
[0052]
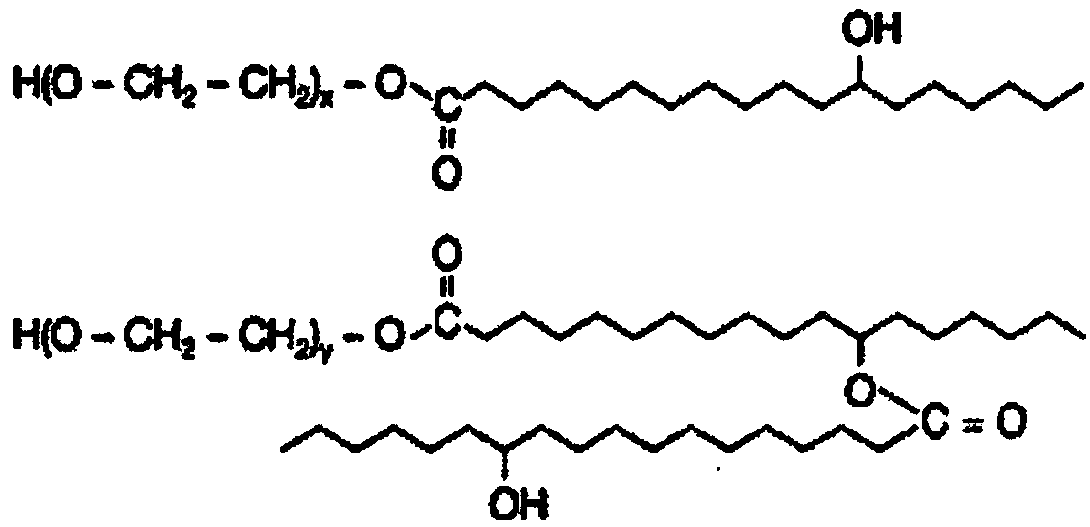
[0053]Preparation process: Add half of the prescribed amount of polyethylene glycol-15-hydroxystearate into the prescribed amount of racemic butylphthalide or L-butylphthalide at 60-65°C, stir and mix thoroughly; then add the prescribed amount Measure one-third of the water for injection and stir to make a milky solution. Dissolve the remaining polyethylene glycol-15-hydroxystearate of the prescription quantity in water for injection of one-third of the prescription quantity, stir, slowly add the milky solution, stir, then add the sodium chloride of the prescription quantity, set Make up to 1000ml, stir, filter with ultrafiltration membrane, fill with nitrogen protection, pack in 100ml per bottle / bag, and sterilize.
Embodiment 2
[0054] Embodiment 2, large volume injection of butylphthalide, comprising racemic butylphthalide or L-butylphthalide, polysorbate-80, sodium chloride, EDTA-2Na, pH regulator and water for injection.
[0055] Prescription 2, calculated in 1000ml
[0056]
[0057] Preparation process: at 60-65°C, add the prescribed amount of polysorbate-80 into the prescribed amount of racemic butylphthalide or L-butylphthalide, stir, and mix thoroughly; then add 70% of the prescribed amount of water for injection, stir, and prepare Get solution, add sodium chloride, EDTA-2Na of recipe quantity, be settled to 1000ml, stir, add pH adjuster (hydrochloric acid and / or sodium hydroxide), adjust pH to 6.5, ultrafiltration membrane ultrafiltration, according to each bottle 100ml per bag, dispensed and sterilized.
Embodiment 3
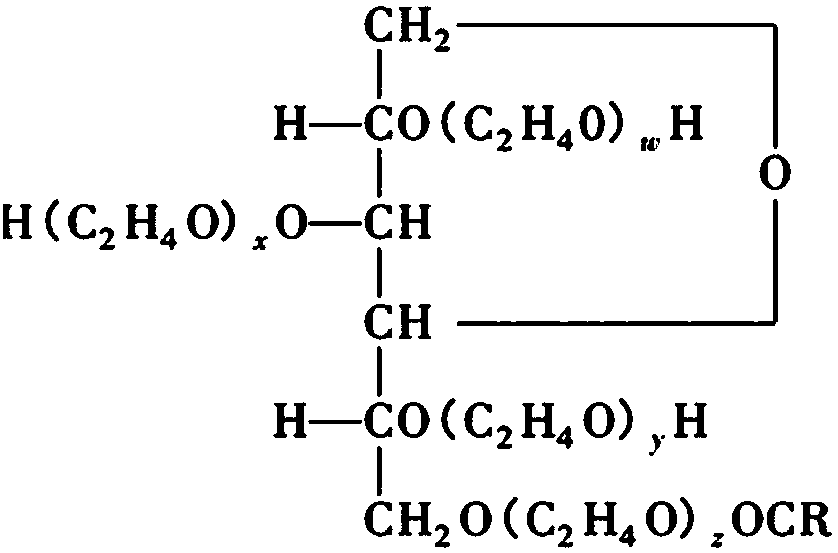
[0058] Embodiment 3, large volume injection of butylphthalide, comprising racemic butylphthalide or L-butylphthalide, poloxamer 188, sodium chloride, pH regulator and water for injection.
[0059] Prescription 3, calculated in 1000ml
[0060]
[0061]
[0062] Preparation process: at room temperature, add the prescribed amount of poloxamer 188 to the prescribed amount of racemic butylphthalide or L-butylphthalide, stir, and mix thoroughly; then add 70% of the prescribed amount of water for injection, and stir to prepare a solution , add the prescribed amount of sodium chloride, set the volume to 1000ml, stir, add pH adjuster (disodium hydrogen phosphate and / or sodium dihydrogen phosphate), adjust the pH to 6.5, ultrafiltration membrane ultrafiltration, nitrogen protection, press 100ml per bottle / bag, sterilized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com