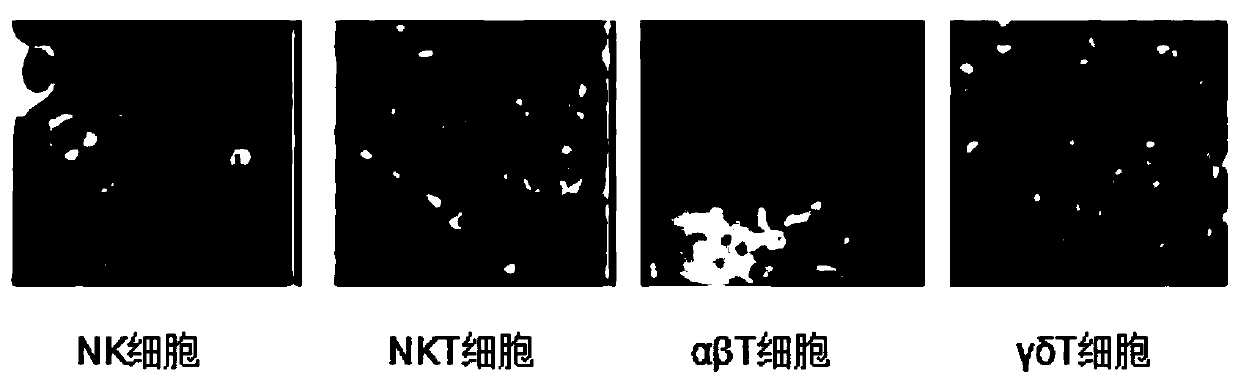
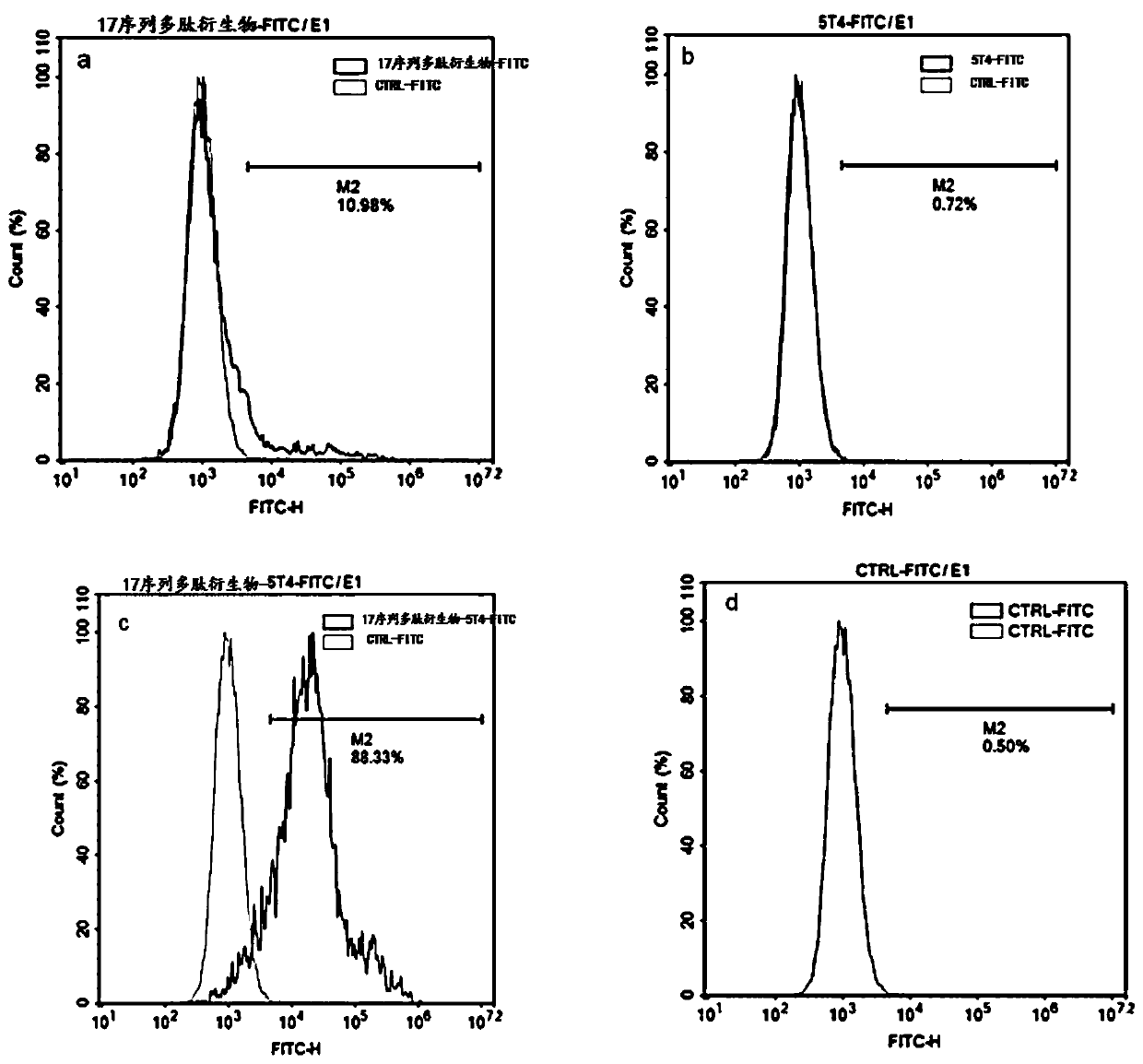
Polypeptide, polypeptide derivative, and polypeptide-antibody complex and preparation and application of polypeptide
A technology of antibody complexes and peptide derivatives, applied in the direction of antibodies, drug combinations, antibody medical components, etc., can solve the problems of low targeting, cumbersome production process, high cost, etc., and achieve enhanced targeting and simple preparation process. , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1 Polypeptide Derivative Synthesis
[0083] The polypeptide derivative of the present invention utilizes the Fmoc solid-phase polypeptide synthesis technology to complete the peptide chain, and the purity is greater than 95% through HPLC detection.
[0084] Synthetic raw materials and related reagents:
[0085] Fmoc-L-Asp(Otbu)-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0086] Fmoc-L-Lys(Boc)-OH, Jill Biochemical (Shanghai) Co., Ltd.
[0087] Fmoc-L-Gly-OH, Jill Biochemical (Shanghai) Co., Ltd.
[0088] Fmoc-Pro-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0089] Fmoc-Ser(Tbu)-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0090] Fmoc-L-Lys(Dde)-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0091] myristic acid,
[0092] Azidoacetic acid NHS active ester
[0093] HBTU (N, N, N', N'-tetramethyl-O-(1H-benzotriazole) urea hexafluorophosphate),
[0094] DIEA (N, N-diisopropylethylamine), DMF (N, N-dimethylformamide),
[0095] DCM (dichloromethane), acetonitril...
Embodiment 2
[0145] Example 2 Synthesis of Polypeptide Derivatives
[0146] The peptide chain is completed by Fmoc solid-phase peptide synthesis technology, and the purity is greater than 95% through HPLC detection.
[0147] Synthetic raw materials and related reagents are the same as in Example 1.
[0148] Fmoc-L-Asp(Otbu)-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0149] Fmoc-L-Lys(Boc)-OH, Jill Biochemical (Shanghai) Co., Ltd.
[0150] Fmoc-L-Gly-OH, Jill Biochemical (Shanghai) Co., Ltd.
[0151] Fmoc-Pro-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0152] Fmoc-Ser(Tbu)-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0153] Fmoc-L-Lys(Dde)-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0154] Cyclooctyne NHS active ester:
[0155] Solid-phase peptide synthesis operation steps:
[0156] 1. Resin swelling
[0157] Weigh 1.0gAM Resin resin with a substitution degree of 0.4mmol / g, put the resin into a reaction tube, add DCM (18ml / g), and shake for 50min.
[0158] 2. Take the first amino...
Embodiment 3
[0202] Example 3 Polypeptide Derivative Synthesis
[0203] The peptide chain is completed by Fmoc solid-phase peptide synthesis technology, and the purity is greater than 95% through HPLC detection.
[0204] Synthetic raw materials and related reagents are the same as in Example 1.
[0205] Fmoc-L-Asp(Otbu)-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0206] Fmoc-L-Lys(Boc)-OH, Jill Biochemical (Shanghai) Co., Ltd.
[0207] Fmoc-L-Gly-OH, Jill Biochemical (Shanghai) Co., Ltd.
[0208] Fmoc-Pro-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0209] Fmoc-Ser(Tbu)-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0210] Fmoc-L-Lys(Dde)-OH, Gill Biochemical (Shanghai) Co., Ltd.
[0211] Solid-phase peptide synthesis operation steps:
[0212] 1. Resin swelling
[0213] Weigh 1.0gAM Resin resin with a substitution degree of 0.4mmol / g, put the resin into a reaction tube, add DCM (18ml / g), and shake for 50min.
[0214] 2. Take the first amino acid
[0215] Filter the solvent through the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com