Cell culture solution and application thereof
A cell culture and stem cell technology, applied in the field of cell culture fluid, can solve the problems of many uncontrollable factors, high cost, high risk, etc., and achieve the effect of promoting proliferation efficiency and normal morphology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This embodiment provides a cell culture solution, the cell culture solution includes apoptotic bodies produced by inducing apoptosis of stem cells in vitro, and the stem cells are umbilical cord mesenchymal stem cells. In some embodiments, the stem cells are bone marrow mesenchymal stem cells, adipose mesenchymal stem cells, dental pulp stem cells, periodontal ligament stem cells, dental follicle stem cells, tooth germ progenitor cells, stem cells from apical papilla, oral epithelium Progenitor / stem cells, gingival-derived mesenchymal stem cells, periosteum-derived stem cells, apoptotic salivary gland-derived stem cells, or any combination thereof.
[0056] The amount of apoptotic bodies added to the cell culture solution described in this example was 2 μg / L. In some embodiments, the amount of apoptotic bodies added in the cell culture medium is 5 μg / L, 10 μg / L, 20 μg / L, 30 μg / L, 40 μg / L, 50 μg / L, 60 μg / L, 70 μg / L L, 80μg / L, 90μg / L, 100μg / L, 110μg / L, 120μg / L, 130μg / L, ...
Embodiment 2
[0077] This example provides the application of the cell culture solution described in Example 1 in maintaining large-scale cultivation of stem cells. The specific application process is as follows:
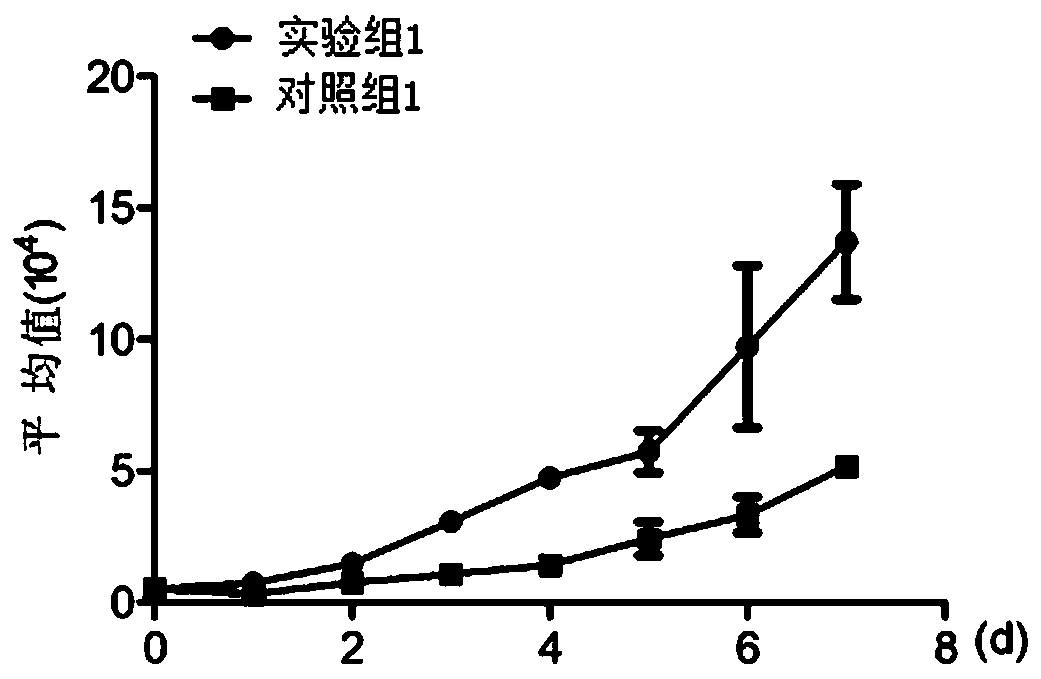
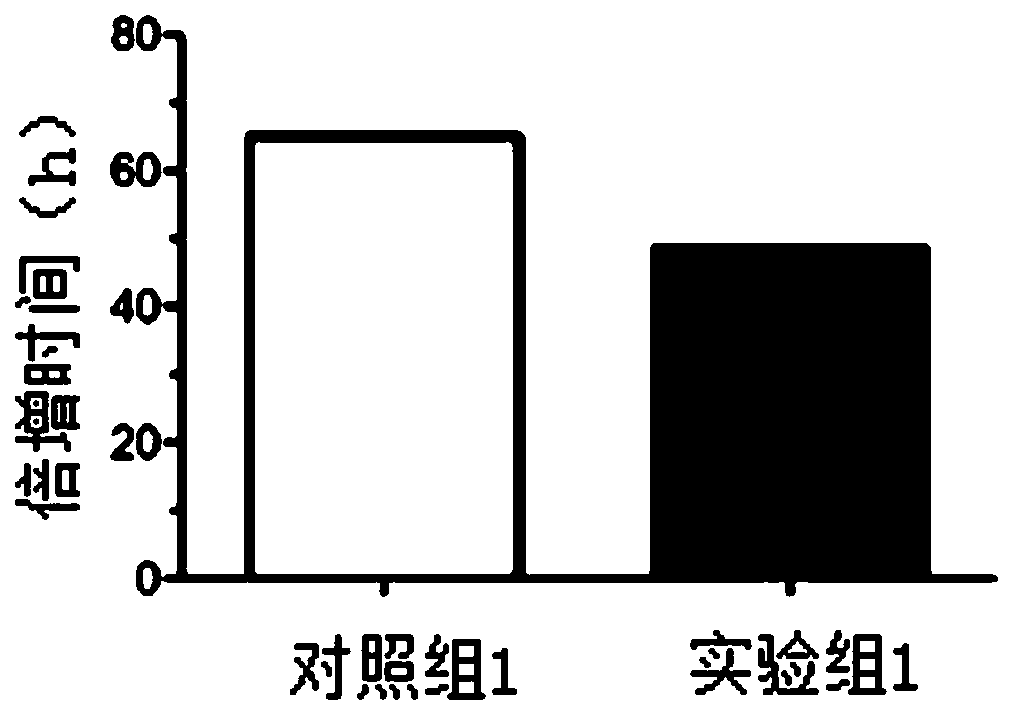
[0078] Cell inoculation: take well-grown mesenchymal stem cells of the 3rd to 20th generation, centrifuge, collect the cell pellet, add the cell culture solution described in Example 1 of the present invention to make a cell suspension, and count them as experimental group 1; Well-grown mesenchymal stem cells of the 20th generation were centrifuged, and the cell pellet was collected, and the culture medium disclosed in patent CN201811341164.5 was added to make a cell suspension, and then counted, which was used as control group 1. According to the results of cell counting, respectively, according to 5 × 10 3 cells / mL for subculture and inoculated in 24-well plates, 1 ml / well. Put in 5% CO after inoculation 2 , 37°C incubator for cultivation. Cell counting: 24 hours after cell ...
Embodiment 3
[0080] Example 3 Verification of osteogenic differentiation function of stem cells obtained after large-scale culture using the cell culture medium provided in the examples of the present invention
[0081]The umbilical cord mesenchymal stem cells cultured in Experimental Group 1 in Example 2 were taken, digested, centrifuged to collect the precipitate, washed with normal saline, and cell suspension was prepared as Experimental Group 2. The umbilical cord mesenchymal stem cells cultured in the control group 1 of Example 2 were taken, digested, centrifuged to collect the precipitate, washed with physiological saline, and prepared into a cell suspension, which was used as the control group 2. After counting, the cells were seeded in a six-well plate, about 2.5×10 per well 5 cells, when the cells grow to 75% ~ 85%, the liquid is replaced with an osteogenic induction solution to continue culturing, wherein the components of the osteogenesis induction solution include 500 parts by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com