Preparation method of cortex albiziae neolignan monomeric compound
A compound, Albizia Julibrissin technology, applied in the field of biochemistry, can solve the problems of unclear pharmacological and pharmacodynamic activities, difficult compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
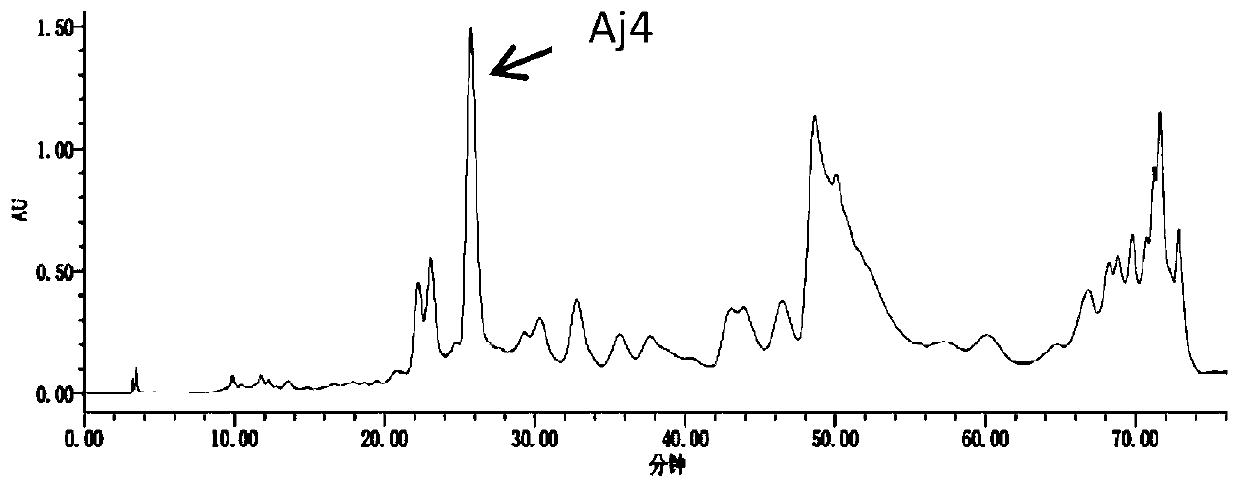
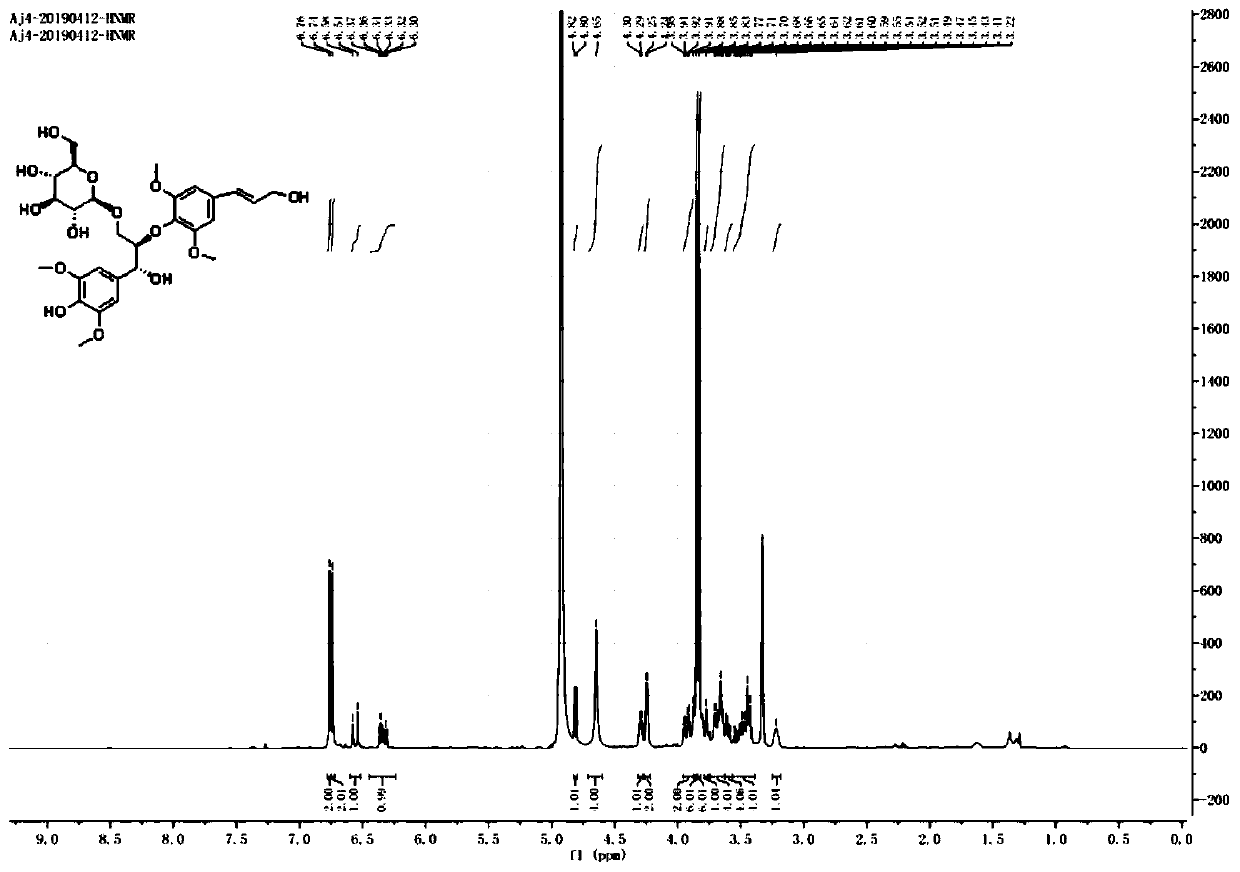
[0047] The preparation of embodiment 1 compound Aj4
[0048](1) Extraction: take 20 kg of dried Albizia Julibrissin skin, pulverize it, use 5 times of 75% ethanol (water), ie 100 L each time, and extract twice at 80° C. for 2 hours each time. The residue of the bark of Albizia Julibrissin was removed by filtration, the 75% ethanol extract of the bark of Albizia Julibis was combined, and freeze-dried to obtain 1.6 kg of crude ethanol extract of the bark of Albizia Julibis. Grind the crude extract and suspend it in 2L deionized water to dissolve it as much as possible. After suspension, extract with ethyl acetate and saturated n-butanol successively. The extraction adopts the principle of adding ethyl acetate and saturated n-butanol several times each time, combining the extracts of ethyl acetate phase and saturated n-butanol phase respectively. Extracts of ethyl acetate and n-butanol fractions were obtained.
[0049] (2) Separation: take 254g of n-butanol, dissolve the suspen...
Embodiment 2
[0063] The preparation of embodiment 2 compound Aj5
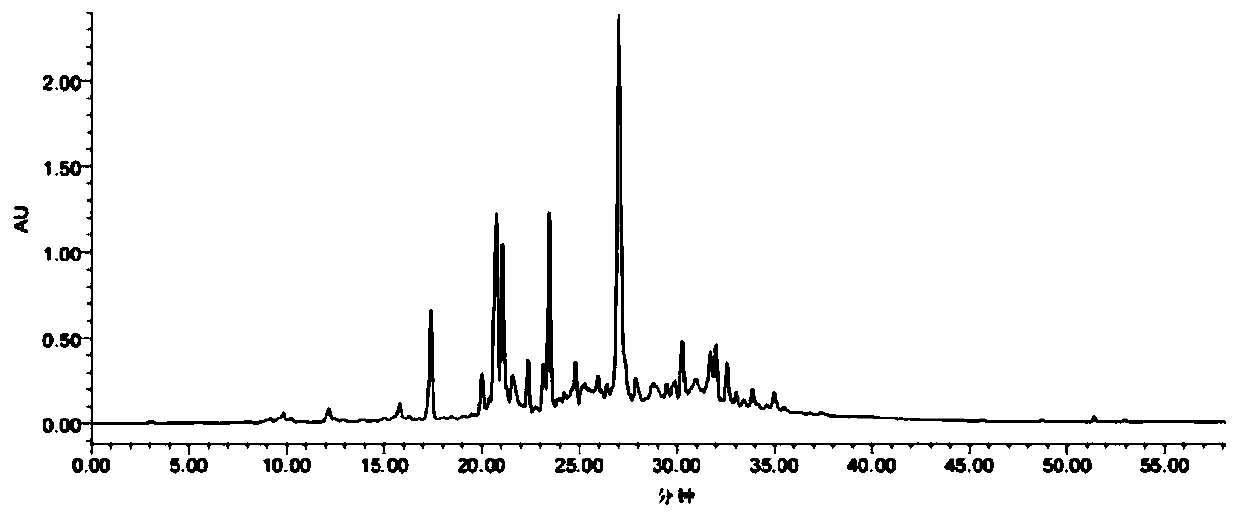
[0064] The step of extraction and separation is with reference to embodiment 1, according to Figure 6 The retention time of each component in the analytical high performance liquid chromatogram, determine the CH 2 Cl 2 :CH 3 The elution condition of the OH=8:1 elution section through the reverse silica gel column (Davisil C18, 50μm) is t R = CH corresponding to 22min, 24min, 26min, 60min 3 OH concentration is 39.8%, 42.9%, 46.2%, 100%, because the diameter of the C18 filler used is 50 μm, so CH 3 The concentration of OH needs to be subtracted by 10%, that is, 29.8%, 32.9%, 36.2%, and 100%. Elution was carried out with methanol and water as the elution phase. according to Figure 6 Determine the elution phase combination as 29% CH 3 OH(H 2 O), 32% CH 3 OH(H 2 O), 36% CH 3 OH(H 2 O), 100% CH 3 OH. Analytical HPLC detection was carried out for each eluted component, and the separation conditions were explored to...
Embodiment 3
[0073] The preparation of embodiment 3 compound Aj6
[0074] Extraction, separation condition are the same as embodiment 2, semi-preparative separation chromatogram is as Figure 11 shown. to 32% CH 3 The OH elution section is subjected to semi-preparative liquid phase separation (the chromatographic column is X-Bridge C18, 5μm, 10×250mm, the flow rate is 4ml / min, and the column temperature is 30°C), such as Figure 15 Shown (UV detection wavelength 290nm), collection retention time t R = The compound at 35.20 min was rotated under reduced pressure at 75°C, the solvent was recovered, and the monomer compound Aj6 was obtained by drying in a vacuum oven.
[0075] Structural identification: Aj6 is a light yellow powder, detected by UPLC-ESI-MS, and its mass spectrum is as follows Figure 12 shown. ESI-MS m / z 617[M+Cl - ], through Monoisotopic Mass, Even Electron Ions determine its molecular formula is C 28 h 38 o 13 .
[0076] Dissolve the sample Aj5 in a nuclear magnet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com