Eribulin and detection method of related substance in preparation containing Eribulin
A detection method and a technology related to substances, which are applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as interference, and achieve the effects of high separation, strong superiority, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Determination of Eribulin Related Substances
[0025] 1. Chromatographic conditions and system suitability experiments
[0026] 1.1 Selection of chromatographic conditions:
[0027] Instrument: Agilent 1260, the optimum column temperature is 35°C, the flow rate is 1.0ml / min, and the detection wavelength is 254nm. Eclipse XDB-C18 column for liquid chromatography (150mm×4.6mm, 5μm), 0.01mol / L NH 4 OAc solution (pH5.0) - acetonitrile or 0.01mol / L NH 4 OAc solution (pH6.0)-methanol is the mobile phase composition, and its optimal ratio is: 10:90 (the mobile phase composition sequence is consistent with the volume ratio sequence). The injection volume is 20 μl.
[0028] Under this chromatographic condition, the main peak of eribulin had a moderate retention time and a good peak shape.
[0029] 1.2 Sensitivity determination
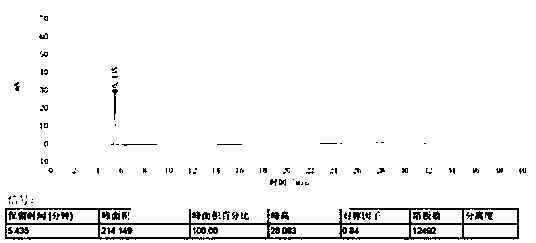
[0030] Take an appropriate amount of eribulin, use the mobile phase to prepare a solution with a concentration of 0.2 mg per 1 ml, an...
Embodiment 2
[0040] Embodiment Two: Determination of Related Substances in Eribulin Mesylate Injection
[0041] Get the test product, accurately measure an appropriate amount (approximately equivalent to eribulin 2 mg), add mobile phase to configure a solution containing 0.2 mg per 1 ml, filter, and take the diluted filtrate as the test product solution. Precisely measure an appropriate amount, add mobile phase to dilute to a solution containing about 2 μg per 1 ml, and use it as a control solution. Under the following selected chromatographic conditions:
[0042] UV detector (Agilent 1260) detection wavelength 254nm, Eclipse XDB-C18 column (150mm×4.6mm, 5 μm), 0.01mol / L NH 4 OAc solution (pH6.0)-acetonitrile composition, the optimal ratio is: 10:90. The column temperature of the chromatographic column is 30°C, and the flow rate is 1.0ml / min. The injection volume is 20 μl. The theoretical plate is not less than 3000 based on Eribulin peak.
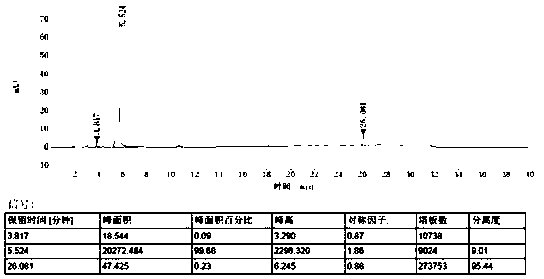
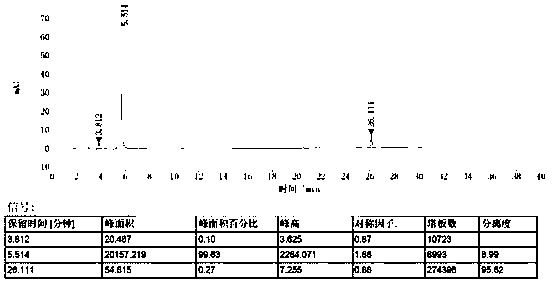
[0043] The measurement results are shown in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com