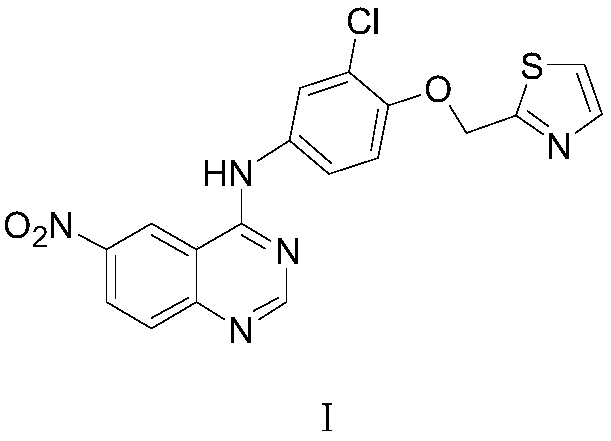
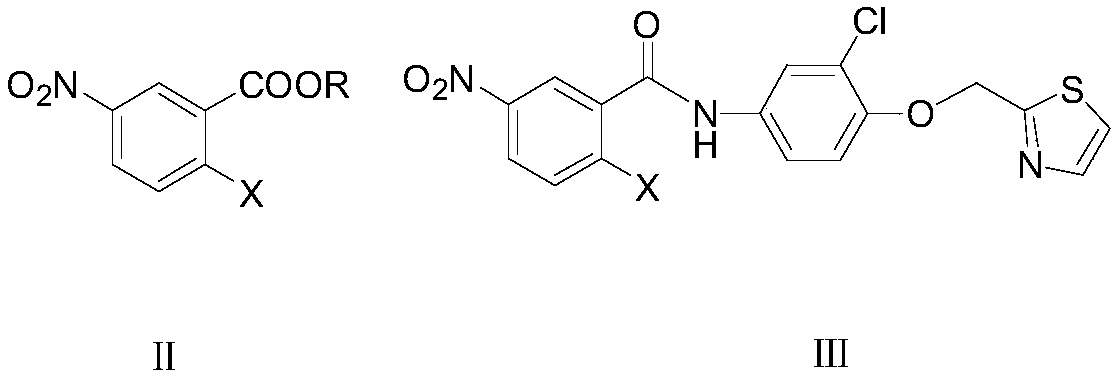
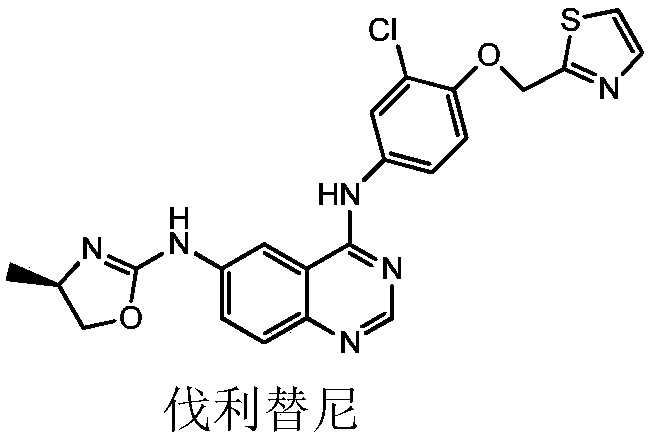
Preparation method of 6-nitro-4-substituted aminoquinazoline derivative
A thiazole and compound technology, applied in the field of pharmaceutical intermediate chemistry, can solve the problems of complicated preparation process, many isomer by-products, unfavorable product purification, valilitinib quality assurance, etc., and achieves high reactivity and stable raw materials. Good performance and specific effect of amidation reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of N-[4-(thiazol-2-yl)methoxy-3-chlorophenyl]-2-chloro-5-nitrobenzamide (Ⅲ1)
[0052] In the 1000 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, add 500 grams of toluene, 43.1 grams (0.2 moles) of methyl 2-chloro-5-nitrobenzoate (Ⅱ1), 48.1 grams (0.2 moles ) 4-(thiazol-2-yl)methoxy-3-chloroaniline, 5.0 g of ammonium chloride, stirred and reacted at 95 to 100° C. for 5 hours, and distilled off the produced methanol. Cool to 20-25°C, add 50 grams of water, separate layers, distill the organic phase, recycle the solvent toluene, and recrystallize the residue with methyl tert-butyl ether to obtain 81.8 grams of N-[4-(thiazol-2-yl)methanol Oxy-3-chlorophenyl]-2-chloro-5-nitrobenzamide (Ⅲ1), yield 96.5%, liquid phase purity 99.7%.
Embodiment 2
[0053] Example 2: Preparation of N-[4-(thiazol-2-yl)methoxy-3-chlorophenyl]-2-bromo-5-nitrobenzamide (Ⅲ2)
[0054] In the 500 milliliter four-neck flask that is connected with stirring, thermometer, reflux condenser, add 250 grams of toluene, 26.0 grams (0.1 moles) of methyl 2-bromo-5-nitrobenzoate (II2), 24.1 grams (0.1 moles ) 4-(thiazol-2-yl)methoxy-3-chloroaniline, 3.5 g of zinc chloride, stirred and reacted at 90 to 95° C. for 5 hours, and distilled off the produced methanol. Cool to 20-25°C, add 20 grams of water, separate layers, distill the organic phase, recover the solvent toluene, and recrystallize the residue with methyl tert-butyl ether to obtain 45.3 grams of N-[4-(thiazol-2-yl)methanol Oxy-3-chlorophenyl]-2-bromo-5-nitrobenzamide (Ⅲ2), yield 96.7%, liquid phase purity 99.3%.
Embodiment 3
[0055] Example 3: Preparation of N-[4-(thiazol-2-yl)methoxy-3-chlorophenyl]-2-chloro-5-nitrobenzamide (Ⅲ1)
[0056] In a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, add 250 g of xylene, 23.0 g (0.1 mole) of ethyl 2-chloro-5-nitrobenzoate (II3), 24.1 g (0.1 mol) 4-(thiazol-2-yl)methoxy-3-chloroaniline, 3.0 g of ammonium chloride, stirred at 105 to 110° C. for 4 hours, and distilled off the ethanol produced. Cool to 20-25°C, add 20 grams of water, separate layers, distill the organic phase, recover the solvent xylene, and recrystallize the residue with methyl tert-butyl ether to obtain 41.1 grams of N-[4-(thiazol-2-yl) Methoxy-3-chlorophenyl]-2-chloro-5-nitrobenzamide (Ⅲ1), yield 96.9%, liquid phase purity 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com