Detection material, preparation method and application of anti-gababr autoantibody in human body fluid
A 17T2A-GABABR, detection material technology, applied in the field of biomedicine, can solve the problems of large demand for antibodies, long detection time, cumbersome steps, etc., to achieve the effect of strong operation skills, short detection cycle, and high detection cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
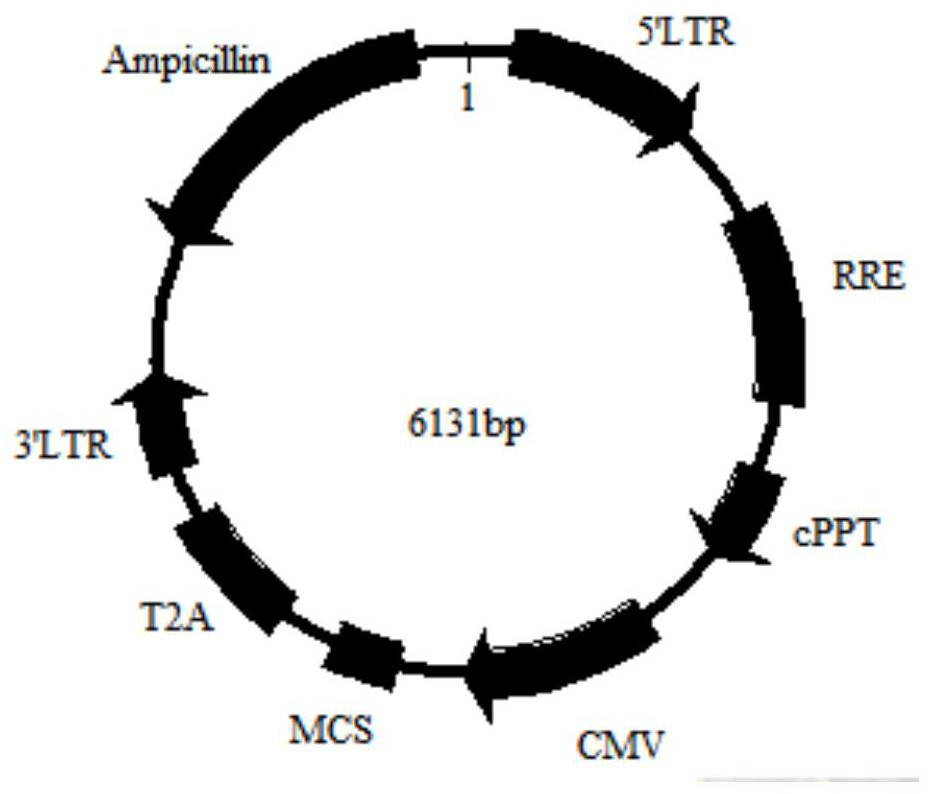
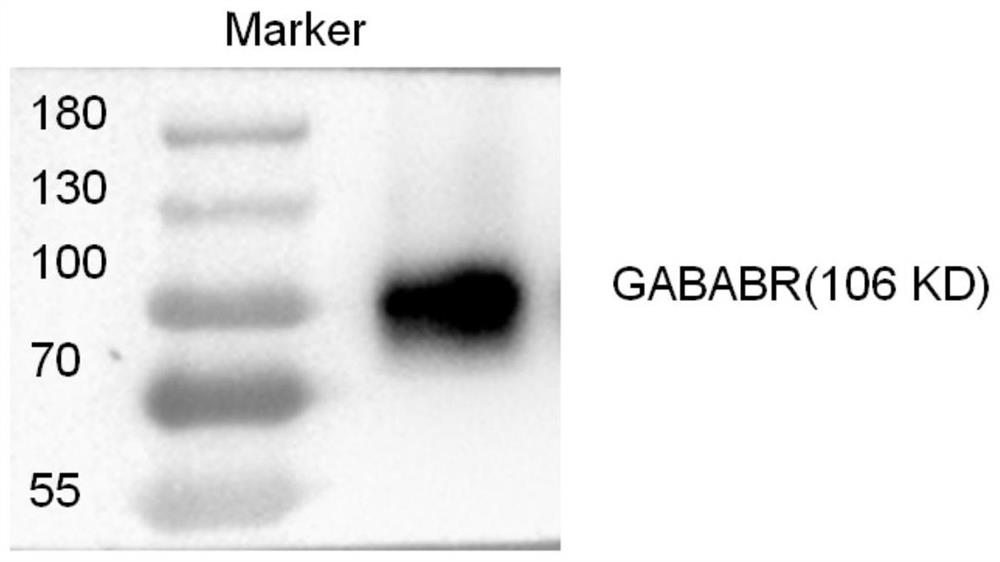
[0039]Step 1, plasmid construction: obtain the CDS sequence of GABABR as the target gene, insert the target gene with restriction sites into the 17T2A plasmid vector to obtain the recombinant plasmid vector 17T2A-GABABR, and extract the plasmid for subsequent experiments after the sequencing is correct. The plasmid vector construction of example specifically comprises the following steps:
[0040] Step 1.1: Obtain the CDS sequence of GABABR as the target gene by PCR method (artificial synthesis method is also optional), and add SalI / NotI restriction sites at both ends of the target gene;
[0041] Step 1.2: Insert the target gene with the restriction site into the 17T2A plasmid vector, the insertion site is SalI / NotI, to obtain a recombinant vector, which is named 17T2A-GABABR;
[0042] Among them, the 17T2A plasmid vector deletes the copGFP element on the basis of the pCDH-CMV-MCS-EF1-copGFP vector, and replaces the FE1 promoter with the T2A element at the same time. The map o...
Embodiment 2
[0065] The difference between this example and Example 1 is that the composite extract in step 3.1 is a mixture of digitonin, TritonX100, NP40, and CHAPS in a volume ratio of 1:1:1:1.
Embodiment 3
[0067] The difference between this example and Example 1 is that the composite extract in step 3.1 is a mixture of digitonin, TritonX100, NP40, and CHAPS in a volume ratio of 1:2:2:1.
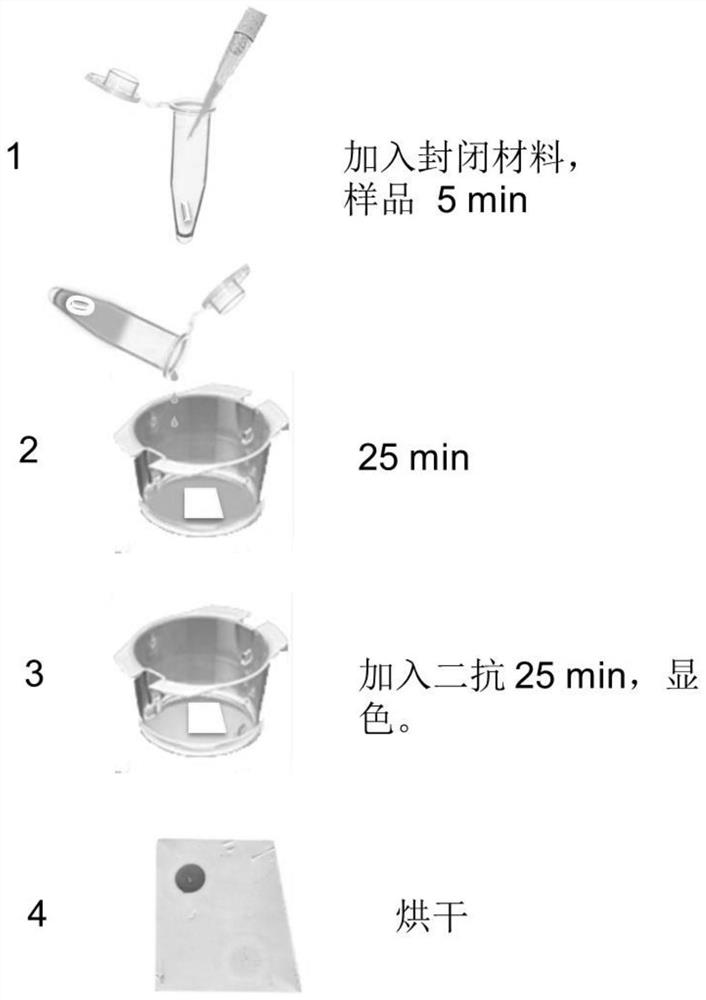
[0068] When the anti-GABABR autoantibody detection material prepared in the above examples is used to detect the GABABR antibody in the sample, the present invention firstly prepares a new blocking material. Since some proteins in human serum and cerebrospinal fluid will non-specifically bind to antigens during the detection process and interfere with the detection results, the present invention uses a sealing material to seal the detection sample, and the implementation effect shows that the sample background can be significantly reduced, as follows A specific example of the preparation of the sealing material of the present invention is given.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com