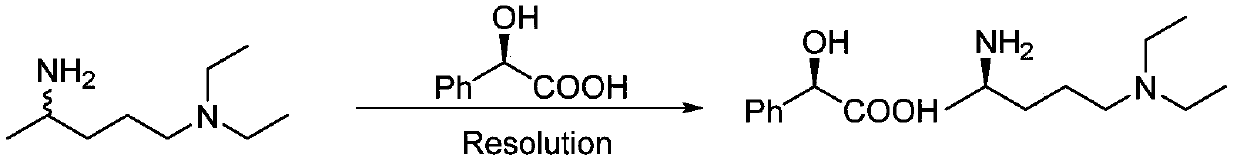
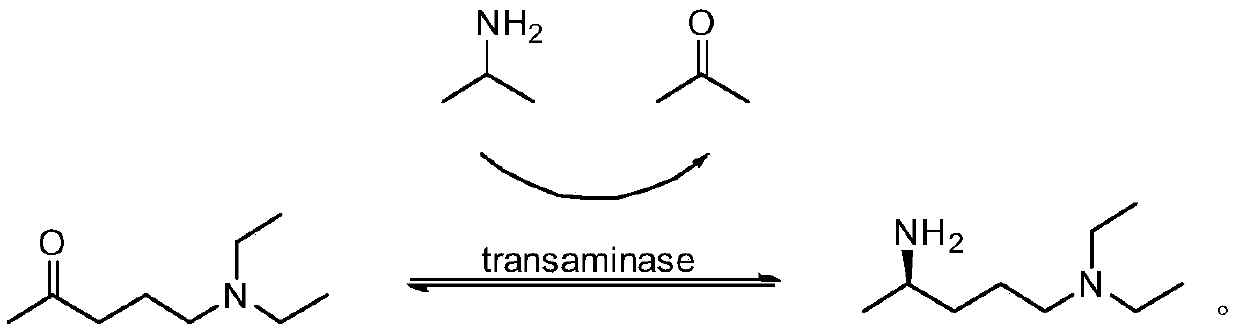
Preparation method of (R)-N1,N1-diethyl-1,4-pentanediamine
A diethyl, -N1 technology, applied in the field of chiral amine preparation, can solve the problems of not finding ω-transaminase, high difficulty in preparation of transaminase, high price, etc., and achieves good industrial application prospects, good stereoselectivity, and simple operation. handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of (R)-N1, the synthetic method of N1-diethyl-1,4-pentanediamine, it comprises the steps:
[0033] S1 Add 157g 5-diethylamino-2-pentanone (1mol, 52.3g / L), 1.50L DMSO and 1.50L water into the reaction vessel; use 100mM phosphate buffer solution to adjust the pH value of the reaction system to 8.5, then add 177g Isopropylamine (3mol), 15g (5g / L) ω-transaminase ATA-117 and 15g (5g / L) coenzyme 5-phosphate-pyridoxal 40 ℃ stirring reaction for 15 hours; Among them, ω-transaminase ATA-117 is the market Purchased, its activity is 300U / L.
[0034] S2 Add 6M hydrochloric acid to the reaction system to adjust the pH value to 2, extract twice with 1500mL dichloromethane; adjust the pH to 12-13 with 6M sodium hydroxide in the aqueous phase, extract three times with 1000mL dichloromethane, combine the organic phases, and wash with water ( 1000mL) once, dried over anhydrous sodium sulfate, concentrated under reduced pressure to dryness to obtain about 150g of crude product;
...
Embodiment 2
[0038] A kind of (R)-N1, the synthetic method of N1-diethyl-1,4-pentanediamine, it comprises the steps:
[0039] S1 Add 157g of 5-diethylamino-2-pentanone (1mol), 1.50L of DMSO and 1.00L of water into the reaction vessel; use 100mM phosphate buffer solution to adjust the pH value of the reaction system to 8.5, then add 177g of isopropylamine (3mol) , 15g ω-transaminase ATA-117 and 15g coenzyme 5-phosphate-pyridoxal were stirred and reacted at 40°C for 15 hours;
[0040] S2 Add 6M hydrochloric acid to the reaction system to adjust the pH value to 2, extract twice with 1500mL dichloromethane; adjust the pH to 12-13 with 6M sodium hydroxide in the aqueous phase, extract three times with 1000mL dichloromethane, combine the organic phases, and wash with water ( 1000mL) once, dried over anhydrous sodium sulfate, concentrated under reduced pressure to dryness to obtain about 135g of crude product;
[0041] The crude product described in S3 was distilled under reduced pressure to obt...
Embodiment 3
[0045] A kind of (R)-N1, the synthetic method of N1-diethyl-1,4-pentanediamine, it comprises the steps:
[0046] S1 Add 157g of 5-diethylamino-2-pentanone (1mol), 1.50L of DMSO and 1.50L of water into the reaction vessel; use 100mM phosphate buffer solution to adjust the pH value of the reaction system to 8.5, then add 118g of isopropylamine (2mol) , 15g ω-transaminase ATA-117 and 15g coenzyme 5-phosphate-pyridoxal were stirred and reacted at 40°C for 15 hours;
[0047]S2 Add 6M hydrochloric acid to the reaction system to adjust the pH value to 2, extract twice with 1500mL dichloromethane; adjust the pH to 12-13 with 6M sodium hydroxide in the aqueous phase, extract three times with 1000mL dichloromethane, combine the organic phases, and wash with water ( 1000mL) once, dried over anhydrous sodium sulfate, concentrated under reduced pressure to dryness to obtain about 150g of crude product;
[0048] The crude product described in S3 was distilled under reduced pressure to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com