Granatilide acetate multivescular liposome and preparation method thereof
A technology of glatiramer acetate and multivesicular liposomes, which is applied in the field of pharmaceutical preparations and can solve the problems of short action time and multiple administrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
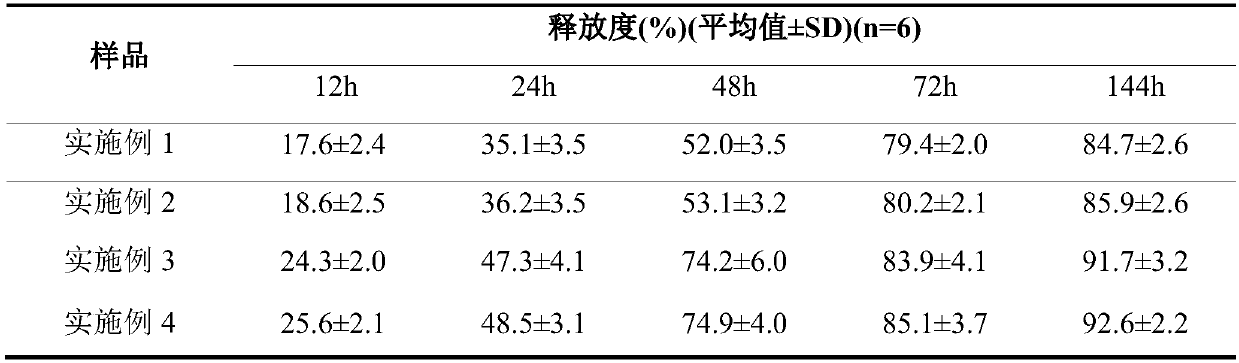
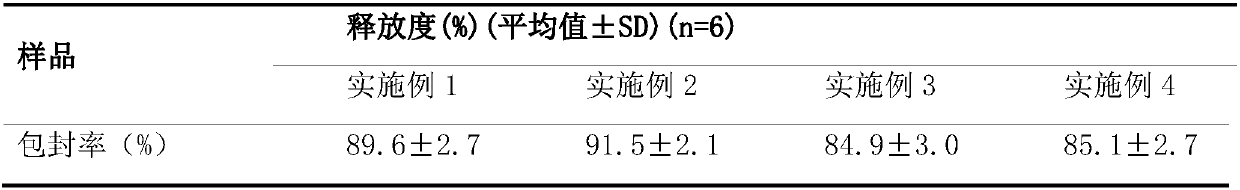
Embodiment 1
[0043] The preparation of embodiment 1 glatiramer multivesicular liposome
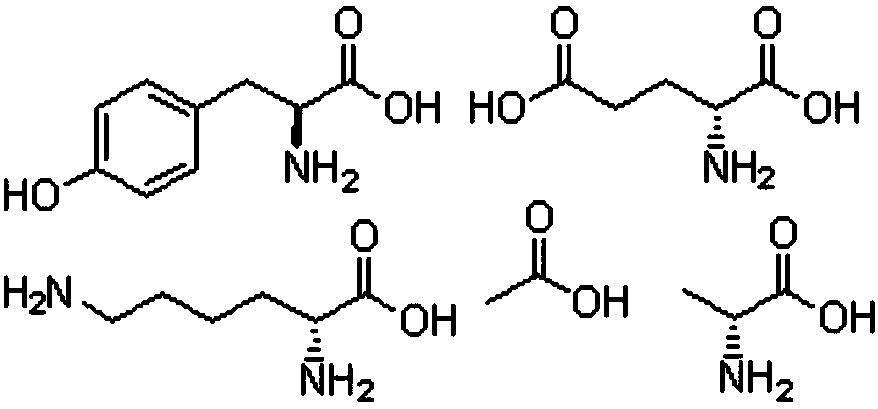
[0044] Dissolve 50 mg of glatiramer acetate in 30 mL of water phase, 5 g of soybean lecithin, 5 g of cholesterol, 0.6 g of phosphatidylserine, and 0.6 g of triolein in 30 mL of chloroform-ether (1:1) (oil phase), The water phase was added into the oil phase, and the vortex mixer was shaken to emulsify for 8 minutes to prepare colostrum. Add the obtained colostrum into the same volume of external water phase, emulsify with a vortex mixer for 1-240s, usually 20-80s. The obtained double emulsion is blown dry with nitrogen gas to obtain the multivesicular liposome. The particle size of the obtained multivesicular liposome is related to the time of secondary emulsification, the longer the time, the smaller the particle size. Centrifuge, remove supernatant, and resuspend multivesicular liposomes with saline.
Embodiment 2
[0045] The preparation of embodiment 2 glatiramer multivesicular liposomes
[0046] Dissolve 200 mg of glatiramer acetate in 100 mL of water phase, 5 g of lecithin, 1.2 g of phosphatidylserine, and 1.1 g of glyceryl tricaprylate, dissolve in 120 mL of chloroform or a mixed solvent of chloroform and ether (oil phase), and dissolve the water phase Add it to the oil phase of the same volume, emulsify for 6-12min with a high-speed disperser at 8000-15000r / min, and prepare colostrum. Add the obtained colostrum into the same volume of external water phase, and emulsify with a high-speed disperser at 4500-5000r / min for 30-120s. The obtained double emulsion is blown dry with nitrogen gas to obtain the multivesicular liposome. Centrifuge, remove supernatant, and resuspend multivesicular liposomes with saline.
Embodiment 3
[0047] The preparation of embodiment 3 glatiramer multivesicular liposomes
[0048] Dissolve 50 mg of glatiramer acetate in 30 mL of water phase, 5 g of soybean lecithin, 5 g of cholesterol, 0.6 g of phosphatidylserine, and 0.1 g of triolein in 30 mL of chloroform-ether (1:1) (oil phase), The water phase was added into the oil phase, and the vortex mixer was shaken to emulsify for 8 minutes to prepare colostrum. Add the obtained colostrum into the same volume of external water phase, emulsify with a vortex mixer for 1-240s, usually 20-80s. The obtained double emulsion is blown dry with nitrogen gas to obtain the multivesicular liposome. The obtained double emulsion is blown dry with nitrogen gas to obtain the multivesicular liposome. Centrifuge, remove supernatant, and resuspend liposomes with saline.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com