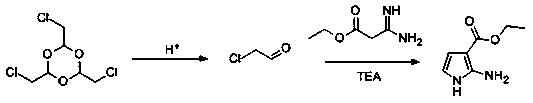
Continuous preparation method for 2-aminopyrrolyl-3-ethyl carboxylate
A technology of ethyl carboxylate and aminopyrrole, which is applied in the field of continuous preparation of ethyl 2-aminopyrrole-3-carboxylate, can solve the problems of low yield of ethyl 2-aminopyrrole-3-carboxylate, etc. Achieve the effects of controllable material ratio, high yield and avoid amplification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] In order to solve the problem of low yield of ethyl 2-aminopyrrole-3-carboxylate, the application provides a continuous preparation method of ethyl 2-aminopyrrole-3-carboxylate, comprising: chloral The solution is continuously fed into the first continuous reactor to carry out continuous acid-catalyzed depolymerization of chloral to obtain a chloroacetaldehyde solution; 3-amino-3-imino ethyl propionate solution, alkali solution and chloroacetaldehyde solution are continuously fed into the second continuous reactor to carry out condensation reaction to obtain ethyl 2-aminopyrrole-3-carboxylate, chloral aldehyde solution, 3-amino-3-iminopropane Ethyl acid solution and alkali solution are organic solutions.
[0030] The continuous preparation method of the present application uses structurally stable chloral as a raw material to continuously prepare commercially available and perishable anhydrous chloroacetaldehyde, and the obtained chloroacetaldehyde is dispersed in the s...
Embodiment 1
[0044] Step1: Add 50 g (0.21mol, 1eq) of chloral into the feeding bottle, then add 500 ml of chloroform, 0.32 g (2.13mmol, 1%eq) of trifluoromethanesulfonic acid, stir until dissolved, A chloral solution was obtained.
[0045] Fill a 30 ml white steel coil with chloroform, adjust the temperature to 155-165 °C (the target temperature is 160 °C), control the pressure at 0.4-0.8 MPa (the target pressure is 0.5-1.0 MPa), and after the temperature stabilizes for 10 min, It can be used for feeding.
[0046] Set the flow rate of the chloral aldehyde solution to 4.34 g / min, the chloral aldehyde solution enters the 30 ml coil tube for reaction, the retention time is 10 min, and the chloroform solution of the chloral aldehyde is obtained, and the NMR internal standard method is used to calculate Chloroacetaldehyde yield is 80%, content 4.9%.
[0047] Step2: Add 15g (0.12mol, 1eq) ethyl 3-amino-3-iminopropionate and 225 mL tetrahydrofuran into the mixing bottle, stir and dissolve to ob...
Embodiment 2
[0056] Step1: Add 50 g (0.21mol, 1eq) of chloral to the feeding bottle, then add 500 ml of ethyl acetate and 0.32 g (2.13mmol, 1%eq) of trifluoromethanesulfonic acid, and stir until Dissolved to obtain chloral aldehyde solution.
[0057] Fill a 30 ml white steel coil with ethyl acetate, adjust the temperature to 155-165°C (the target temperature is 160°C), control the pressure at 0.4-0.8 MPa (the target pressure is 0.5-1.0 MPa), and keep the temperature stable for 10 min After that, the feeding can be carried out.
[0058] The flow rate of the chloral solution is set as 2.72g / min, and the chloral solution enters a 30 ml coil tube for reaction, and the retention time is 10 min to obtain the ethyl acetate solution of chloroacetaldehyde, chloroacetaldehyde The yield is 73%, and the content is 7.2%.
[0059] Step2: Add 15g (0.12mol, 1eq) ethyl 3-amino-3-iminopropionate and 225 mL tetrahydrofuran into the mixing bottle, stir and dissolve to obtain ethyl 3-amino-3-iminopropionate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com