Low-oxygen-responsive polyamino acid-PEG stereo drug-loaded micelle and preparation method thereof
A technology of polyamino acid and drug-loaded micelles, applied in the field of biomedical materials, can solve the problems of less research on polymer micelles, achieve good dispersion and solubility, reduce toxic and side effects, and reduce distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
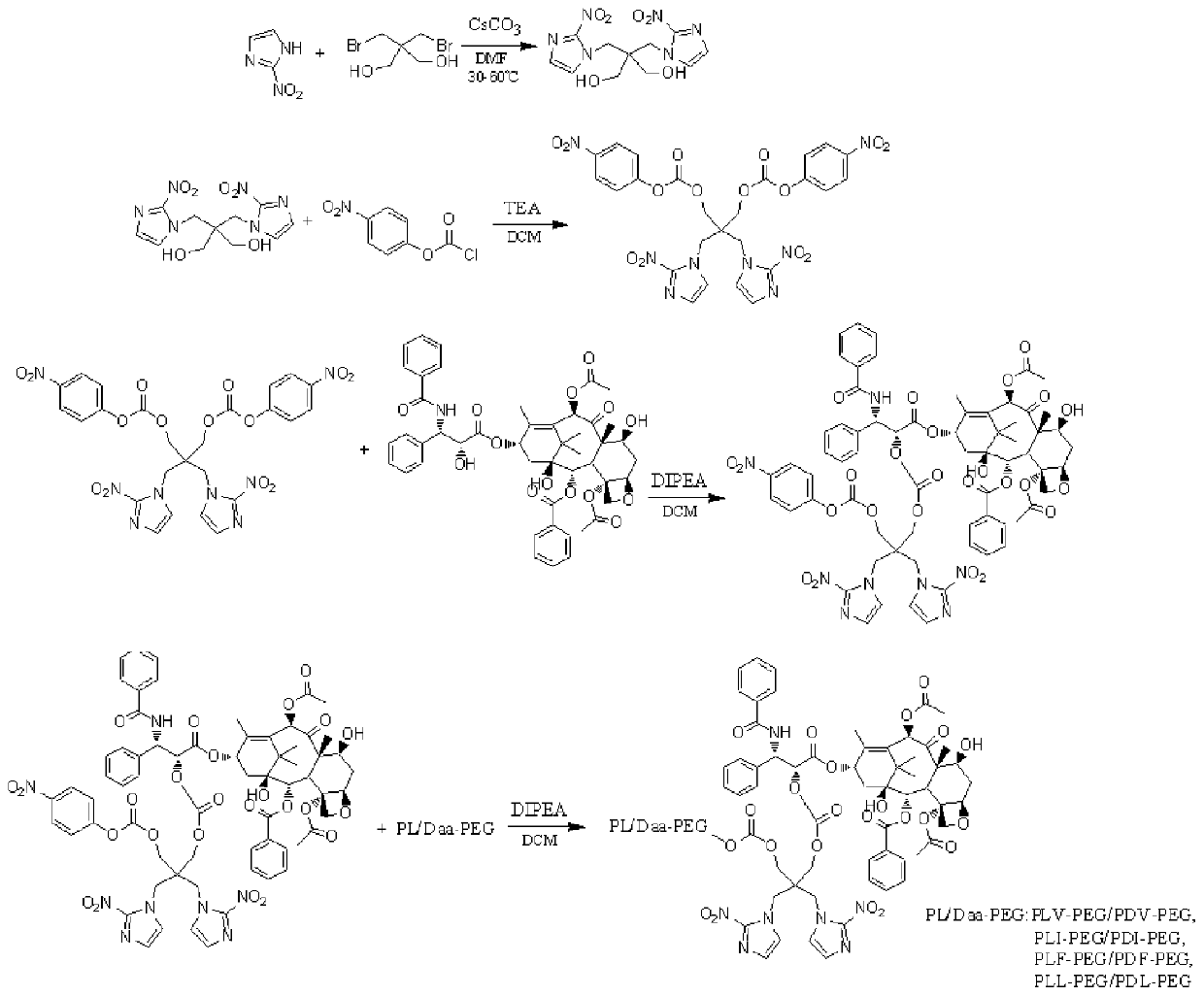
[0033] according to figure 1 The synthetic route shown is the preparation of hypoxia-responsive polyamino acid-PEG stereotactic drug-loaded micelles:
[0034] Step 1: In 6ml DMF, add 0.5g 2-nitroimidazole, 0.4g dibromoneopentyl glycol and 0.8g cesium carbonate, react at 50°C for 12h, after the reaction is completed, distill and concentrate under reduced pressure, and the concentrated silica gel column layer Analyze and separate to obtain 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl]-1,3-propanediol;
[0035] Step 2: In 50ml DCM, add 0.5g 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl]-1,3-propanediol, 0.6g p-nitrobenzoyl chloride and 0.6ml triethylamine, reacted at room temperature for 12h, concentrated by distillation under reduced pressure after the reaction, the concentrate was separated by silica gel column chromatography to obtain 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl ]propane-1,3-diylbis(4-nitrophenyl)carbonate;
[0036] Step 3: In 5ml DCM, add 0.5g 2,2-bis[(2-nitro-1H-imida...
Embodiment 2
[0039] Step 1: In 5ml DMF, add 0.45g 2-nitroimidazole, 0.26g dibromoneopentyl glycol and 0.65g cesium carbonate, react at 50°C for 12h, after the reaction is completed, distill and concentrate under reduced pressure, and concentrate the silica gel column layer Analysis and separation obtained 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl]-1,3-propanediol;
[0040] Step 2: In 30ml DCM, add 0.32g 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl]-1,3-propanediol, 0.37g p-nitrobenzoyl chloride and 0.40ml triethylamine, reacted at room temperature for 12h, concentrated by distillation under reduced pressure after the reaction, the concentrate was separated by silica gel column chromatography to obtain 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl ]propane-1,3-diylbis(4-nitrophenyl)carbonate;
[0041] Step 3: In 2ml DCM, add 0.16g 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl]propane-1,3-diylbis(4-nitrophenyl ) carbonate, 0.21g paclitaxel and 0.12ml N,N-diisopropylethylamine, react at room temperature f...
Embodiment 3
[0044] Step 1: In 8ml DMF, add 0.67g 2-nitroimidazole, 0.52g dibromoneopentyl glycol and 0.97g cesium carbonate, react at 50°C for 12h, after the reaction is completed, distill and concentrate under reduced pressure, and the concentrated silica gel column layer Analysis and separation obtained 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl]-1,3-propanediol;
[0045] Step 2: In 60ml DCM, add 0.65g 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl]-1,3-propanediol, 0.74g p-nitrobenzoyl chloride and 0.80ml triethylamine, reacted at room temperature for 12h, concentrated by distillation under reduced pressure after the reaction, the concentrate was separated by silica gel column chromatography to obtain 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl ]propane-1,3-diylbis(4-nitrophenyl)carbonate;
[0046] Step 3: In 8ml DCM, add 0.65g 2,2-bis[(2-nitro-1H-imidazol-1-yl)methyl]propane-1,3-diylbis(4-nitrophenyl ) carbonate, 0.85g paclitaxel and 0.25ml N,N-diisopropylethylamine, react at room temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com