Ketotifen fumarate tablet and application thereof
A technology for ketotifen fumarate and tablets, which is applied in the fields of pill delivery, medical preparations containing active ingredients, respiratory diseases, etc., and can solve the problems of decreased dissolution rate, poor stability, and insufficient mixing uniformity of tablets, etc. problem, to achieve the effect of dissolution stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A kind of ketotifen fumarate tablet, its prescription (5000 tablets) is shown in Table 1:
[0040] Table 1
[0041]
Embodiment 2
[0043] The preparation process of ketotifen fumarate tablet:
[0044] 1. Weighing
[0045] Weigh ketotifen fumarate, lactose monohydrate, cornstarch, and pregelatinized starch according to the prescription, and set aside.
[0046] 2. mix
[0047] Put 30% lactose monohydrate and ketotifen fumarate in sequence in a wet granulator for mixing, rotation speed: stirring 200rpm, mixing time: 5min;
[0048] Continue to add 30% lactose, rotation speed: stirring at 200rpm, chopping at 300rpm, mixing for 10min, taking samples from the upper, middle and lower parts respectively, and the mixing uniformity RSD should be ≤3%;
[0049] Add the remaining amount of lactose, pregelatinized starch, and cornstarch. Speed: Stir at 200rpm, chop at 300rpm, mix for 5min, stir at 400rpm, chop at 1200rpm, mix for 5min, take samples from the upper, middle, and lower parts for testing, and mix Uniformity RSD should be ≤ 3%.
[0050] 3. Preparation of 6% hydroxypropyl cellulose aqueous solution
[005...
Embodiment 3
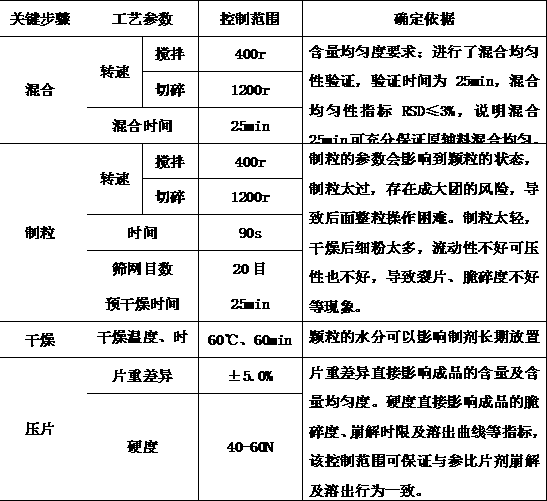
[0066] The key steps of this product are mixing, granulating, drying, total blending and tabletting. The key steps and process parameters are shown in Table 2.
[0067] Table 2
[0068]
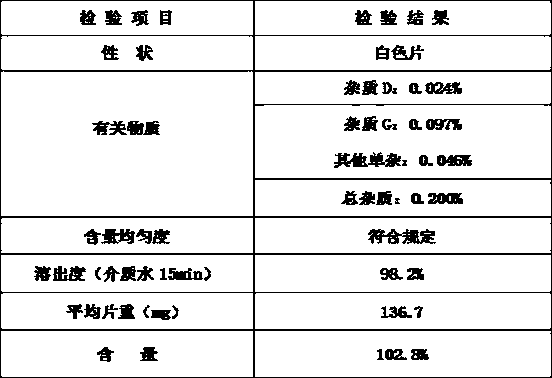
[0069] Table 3 Test results of finished products
[0070]
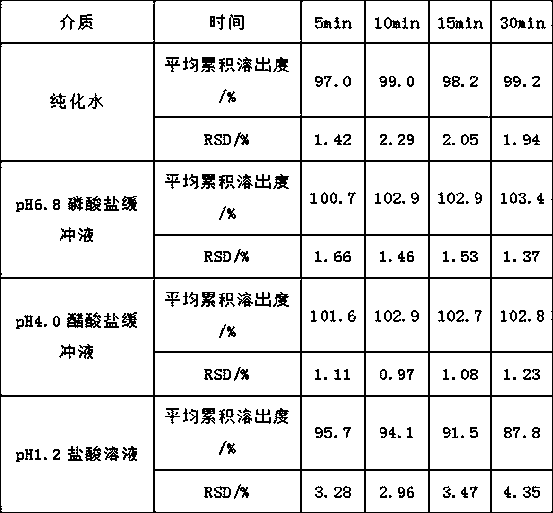
[0071]Table 4 Dissolution curve results of the finished product in four media
[0072]
[0073] Stability test under high temperature and high humidity conditions:
[0074] The product was placed in a drug stability test chamber (temperature 40°C ± 2°C, relative humidity 75% ± 5%) for 3 months, with pH 1.2 hydrochloric acid solution as the dissolution medium, the results showed that: under the accelerated test conditions There is no change in the dissolution rate of this product after placing it for 3 months, which shows that the tablet prepared according to the technical scheme of the present invention is stable in dissolution and is not affected by high temperature and high humidity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com