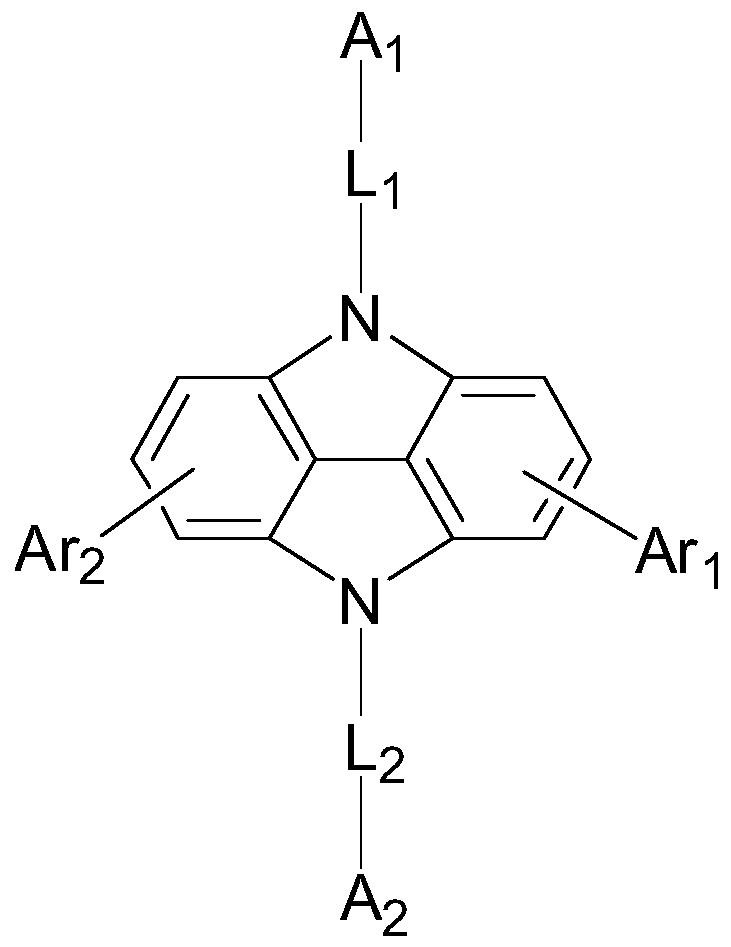
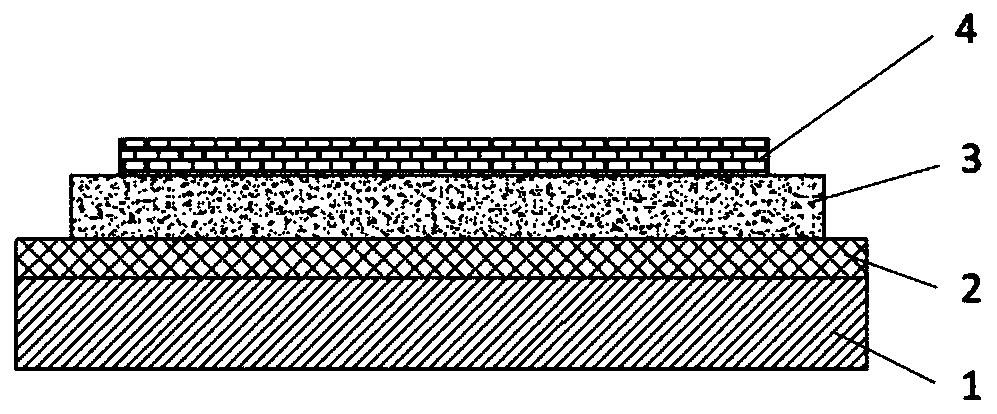
Compound, display panel and display device
A technology for display panels and compounds, applied in the field of compounds with TADF properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Synthesis of Compound P1
[0069]
[0070] Dissolve S2 (1mmol) and S18 (2mmol) in toluene solvent under nitrogen protection, add Pd(PPh 3 ) 4 , Refluxed at 120°C for 6h. After the reaction was completed, it was cooled to room temperature and the crude product was collected. The crude product was purified by silica gel chromatography using a mixed solvent of n-hexane:chloroform (5:1) as the eluent, and finally purified to obtain solid P1 (0.88 mmol, 88%).
[0071] MALDI-TOF MS: C 48 h 30 B 2 N 2 o 2 , m / z calculated: 688.39; measured: 688.25.
[0072] Elemental analysis results: C, 83.75; H, 4.39; B, 3.14; N, 4.07; O, 4.65; Measured values: C, 83.65;
Embodiment 2
[0074] Synthesis of Compound P2
[0075]
[0076] Dissolve S1 (1mmol) in o-dichlorobenzene solvent under nitrogen protection, add PPh 3 , heated to reflux, and reacted for 8h. After the reaction was completed, it was cooled to room temperature, all the solvent was distilled off under reduced pressure, and the crude product was collected. The crude product was purified by silica gel chromatography using a mixed solvent of n-hexane:chloroform (5:1) as the eluent, and finally purified to obtain solid S2 (0.92 mmol, 92%).
[0077] MALDI-TOF MS: C 12 h 8 N 2 , m / z calculated: 180.21; measured: 180.07.
[0078]
[0079] Dissolve S2 (1mmol) and S3 (2mmol) in toluene solvent under nitrogen protection, add Pd(PPh 3 ) 4 , Refluxed at 120°C for 6h. After the reaction was completed, it was cooled to room temperature and the crude product was collected. The crude product was purified by silica gel column chromatography, using a mixed solvent of n-hexane:chloroform (5:1) as e...
Embodiment 3
[0083] Synthesis of Compound P4
[0084]
[0085] Dissolve S2 (1mmol) and S19 (2mmol) in toluene solvent under nitrogen protection, add Pd(PPh 3 ) 4 , Refluxed at 120°C for 6h. After the reaction was completed, it was cooled to room temperature and the crude product was collected. The crude product was purified by silica gel column chromatography, using a mixed solvent of n-hexane:chloroform (5:1) as eluent, and finally purified to obtain solid P4 (0.88mmol, 88%).
[0086] MALDI-TOF MS: C 48 h 28 N 2 o 4 S 2 , m / z calculated: 760.88; measured: 760.15.
[0087] Elemental analysis results: C, 75.77; H, 3.71; N, 3.68; O, 8.41; S, 8.43; measured values: C, 75.67;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com