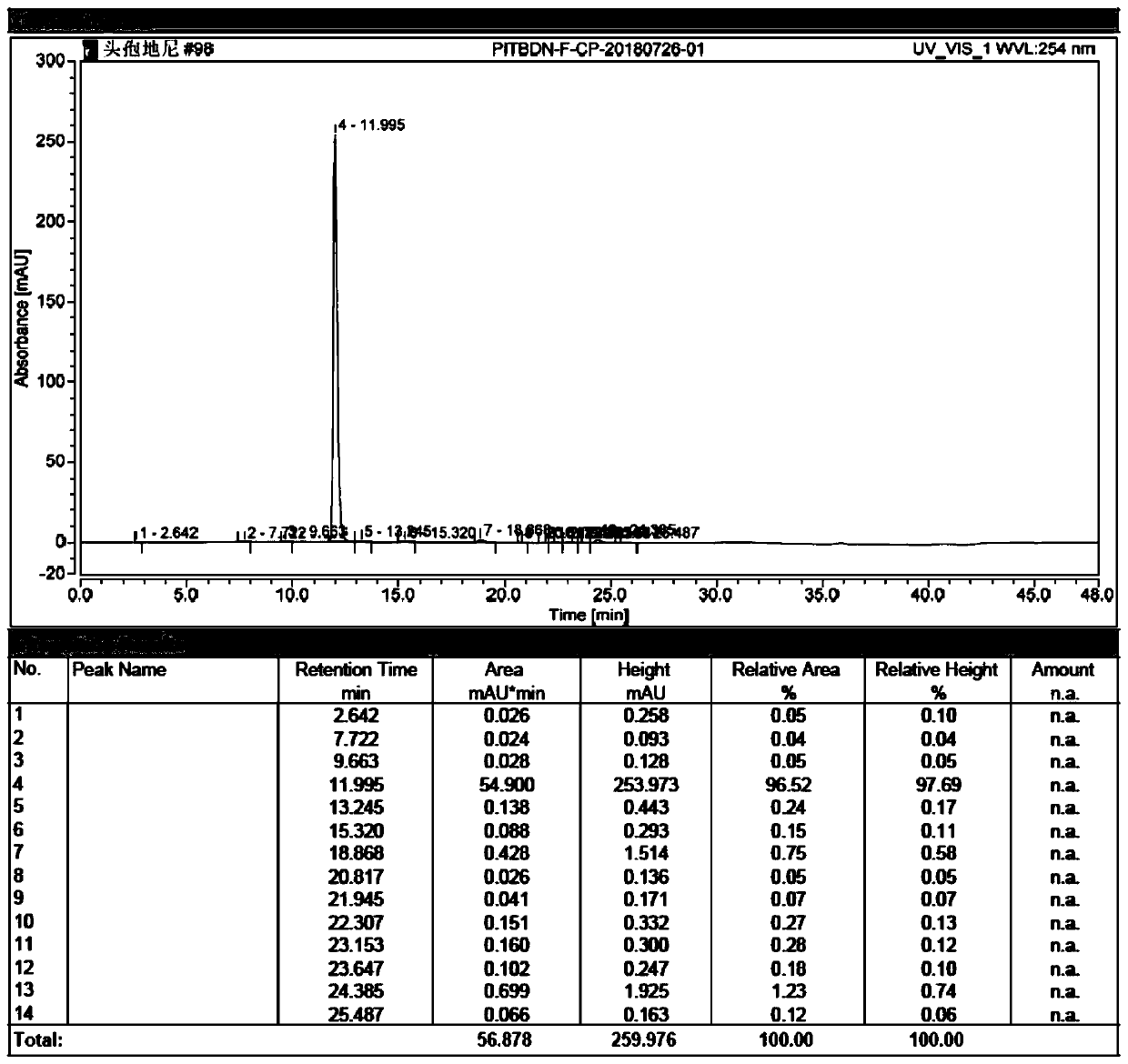
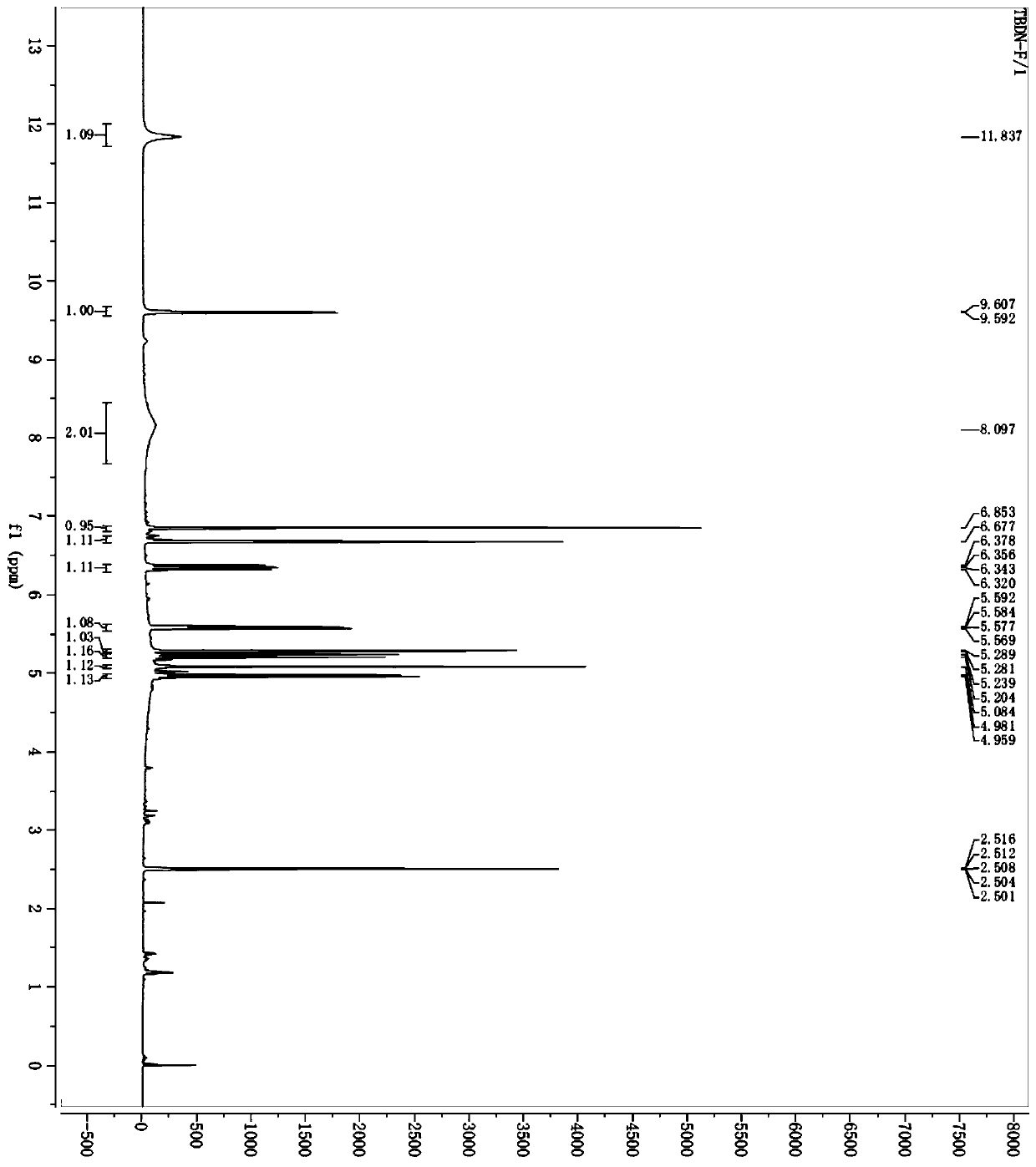
Preparation method of cefdinir impurity F
A cefdinir and impurity technology, which is applied in the field of medicinal chemistry, can solve the problems of affecting drug safety, difficult to obtain monomer impurities, carcinogenic teratogenicity of impurities, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of cefdinir impurity F, comprising the following steps:
[0030] (1) At room temperature, weigh 10 g of cefdinir, add 100 mL of acetonitrile, start stirring, add 10 mL of triethylamine dropwise, start the reaction, and react at 20°C for 6 hours;
[0031] (2) After the reaction is finished, adjust the pH to 5 with 0.1mol / L hydrochloric acid to obtain a mixed solution;
[0032] (3) Put the mixed solution obtained in step (2) on a 400mL C18 column for column chromatography separation, and successively use 1%, 3%, and 6% acetonitrile aqueous solutions to elute 2.5L, and the separated fractions are frozen After drying, the cefdinir impurity F with a purity of more than 90% was obtained, and the yield was 30.2%.
Embodiment 2
[0034] A preparation method of cefdinir impurity F, comprising the following steps:
[0035] (1) At room temperature, weigh 10 g of cefdinir, add 50 mL of acetonitrile, start stirring, add 1 mL of triethylamine dropwise, start the reaction, and react at 5°C for 6 h;
[0036] (2) After the reaction is finished, adjust the pH to 5 with 0.1mol / L hydrochloric acid to obtain a mixed solution;
[0037] (3) Put the mixed solution obtained in step (2) on a 400mL C18 column for column chromatography separation, and successively use 1%, 3%, and 6% acetonitrile aqueous solutions to elute 2.5L, and the separated fractions are frozen After drying, the cefdinir impurity F with a purity of more than 90% was obtained, and the yield was 32.7%.
Embodiment 3
[0039] A preparation method of cefdinir impurity F, comprising the following steps:
[0040] (1) At room temperature, weigh 10g of cefdinir, add 150mL of acetonitrile, start stirring, add 5mL of triethylamine dropwise, start the reaction, and react at 10°C for 7h;
[0041] (2) After the reaction is finished, adjust the pH to 5 with 0.1mol / L hydrochloric acid to obtain a mixed solution;
[0042] (3) Put the mixed solution obtained in step (2) on a 400mL C18 column for column chromatography separation, and successively use 1%, 3%, and 6% acetonitrile aqueous solutions to elute 2.5L, and the separated fractions are frozen After drying, the cefdinir impurity F with a purity of more than 90% was obtained, and the yield was 35.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com