Phenothiazine dyes and application thereof in dye-sensitized solar cells
A phenothiazine and dye technology is applied to organic photoelectric functional materials and their application in solar cells to achieve the effects of improving morphology, enhancing light absorption capacity and high photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the synthesis of CzPTZ-2 dyestuff
[0030] The phenothiazine dye in this example is CzPTZ-2, and the specific preparation process is as follows:
[0031] 1. Preparation of phenothiazine π bridge:
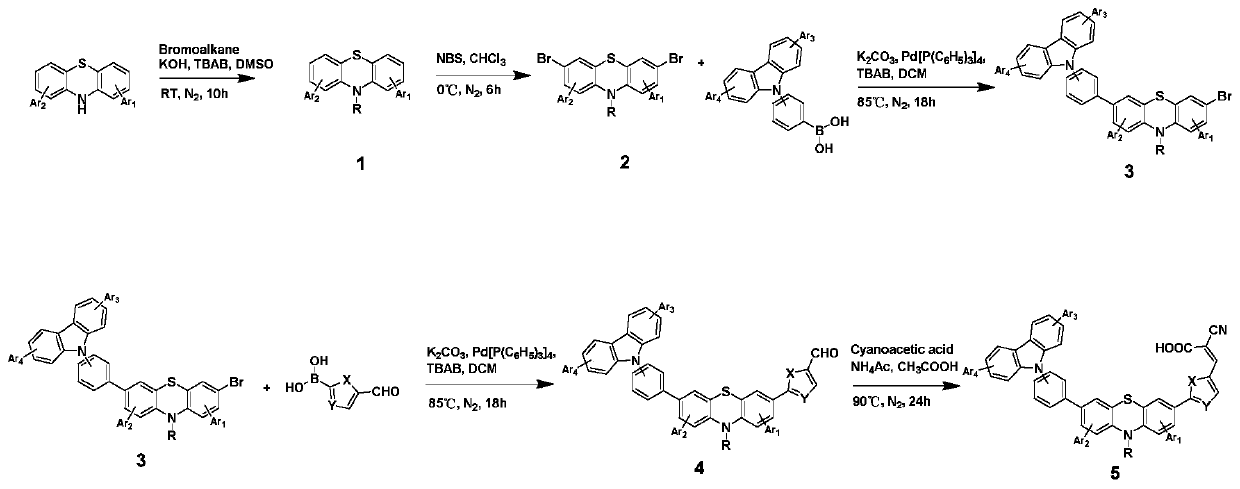
[0032] Dissolve phenothiazine, hexyl bromide, potassium hydroxide and tetrabutylammonium bromide (TBAB) in dimethyl sulfoxide (DMSO) at a molar ratio of 1:2:3:0.5, and react at room temperature under nitrogen protection After 10 hours, the reaction was quenched and the organic phase was extracted, separated and purified by column chromatography to obtain the intermediate product 1, namely N-hexylphenothiazine; the intermediate product 1 was dissolved in chloroform solvent, and bromosuccinyl was added in three batches Amine (NBS) with a molar ratio of 1:3 was reacted in an ice bath under the protection of nitrogen for 6 hours, the reaction was quenched and the organic phase was extracted, separated and purified by column chromatography to obtain the intermediate...
Embodiment 2
[0040] Embodiment 2: the synthesis of CzPTZ-3 dyestuff
[0041] The phenothiazine dye in this example is CzPTZ-3, and the specific preparation process is as follows:
[0042] 1. Preparation of phenothiazine π bridge:
[0043] Dissolve phenothiazine, hexyl bromide, potassium hydroxide and tetrabutylammonium bromide (TBAB) in dimethyl sulfoxide (DMSO) at a molar ratio of 1:2:3:0.5, and react at room temperature under nitrogen protection After 10 hours, the reaction was quenched and the organic phase was extracted, separated and purified by column chromatography to obtain the intermediate product 1, namely N-hexylphenothiazine; the intermediate product 1 was dissolved in chloroform solvent, and bromosuccinyl was added in three batches Amine (NBS) with a molar ratio of 1:3 was reacted in an ice bath under the protection of nitrogen for 6 hours, the reaction was quenched and the organic phase was extracted, separated and purified by column chromatography to obtain the intermediate...
Embodiment 3
[0051] Embodiment 3: Study the light absorption characteristics of dyes CzPTZ-2 and CzPTZ-3
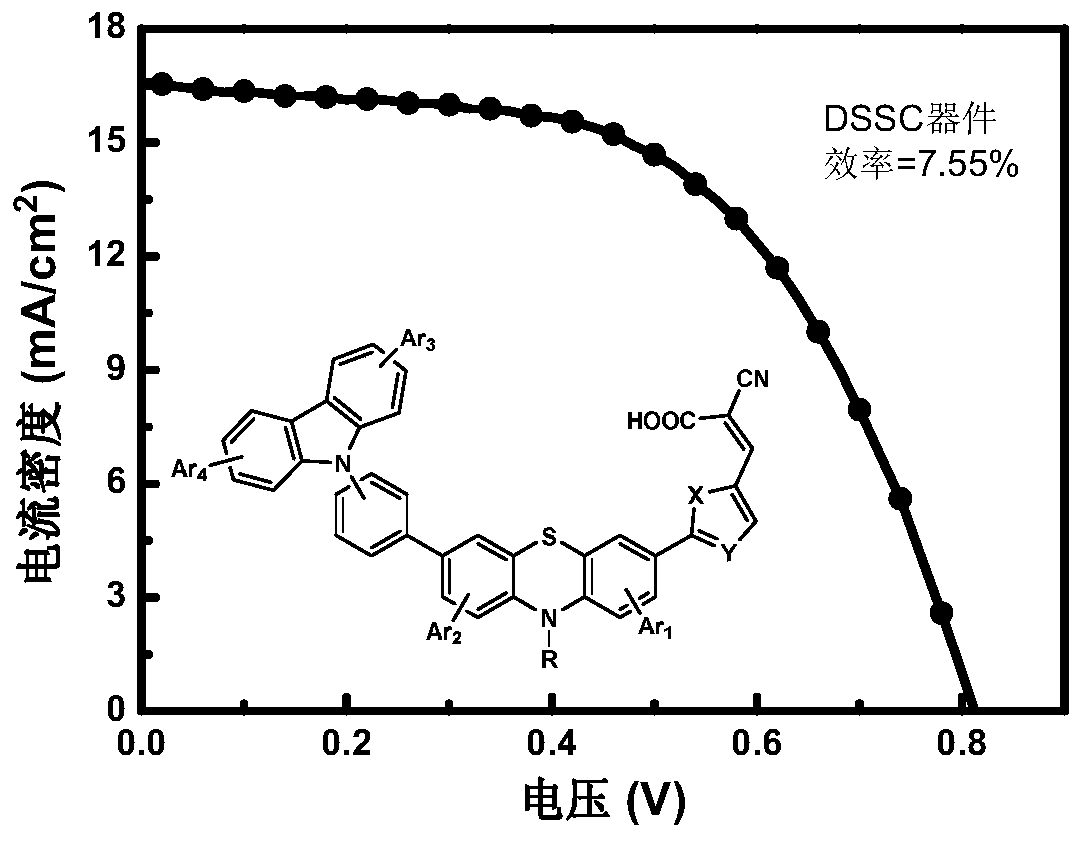
[0052] Configure 100mL concentration as 10 -5 The DCM solution of CzPTZ-2 and CzPTZ-3 of mol / L, test its ultraviolet-visible light absorption spectrum, see appendix Figure 5 . It can be seen from the figure that the intramolecular charge transfer absorption peaks of the two dyes are both at 479nm, and the cut-off wavelength is about 600nm, which proves that they have good absorption of sunlight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com