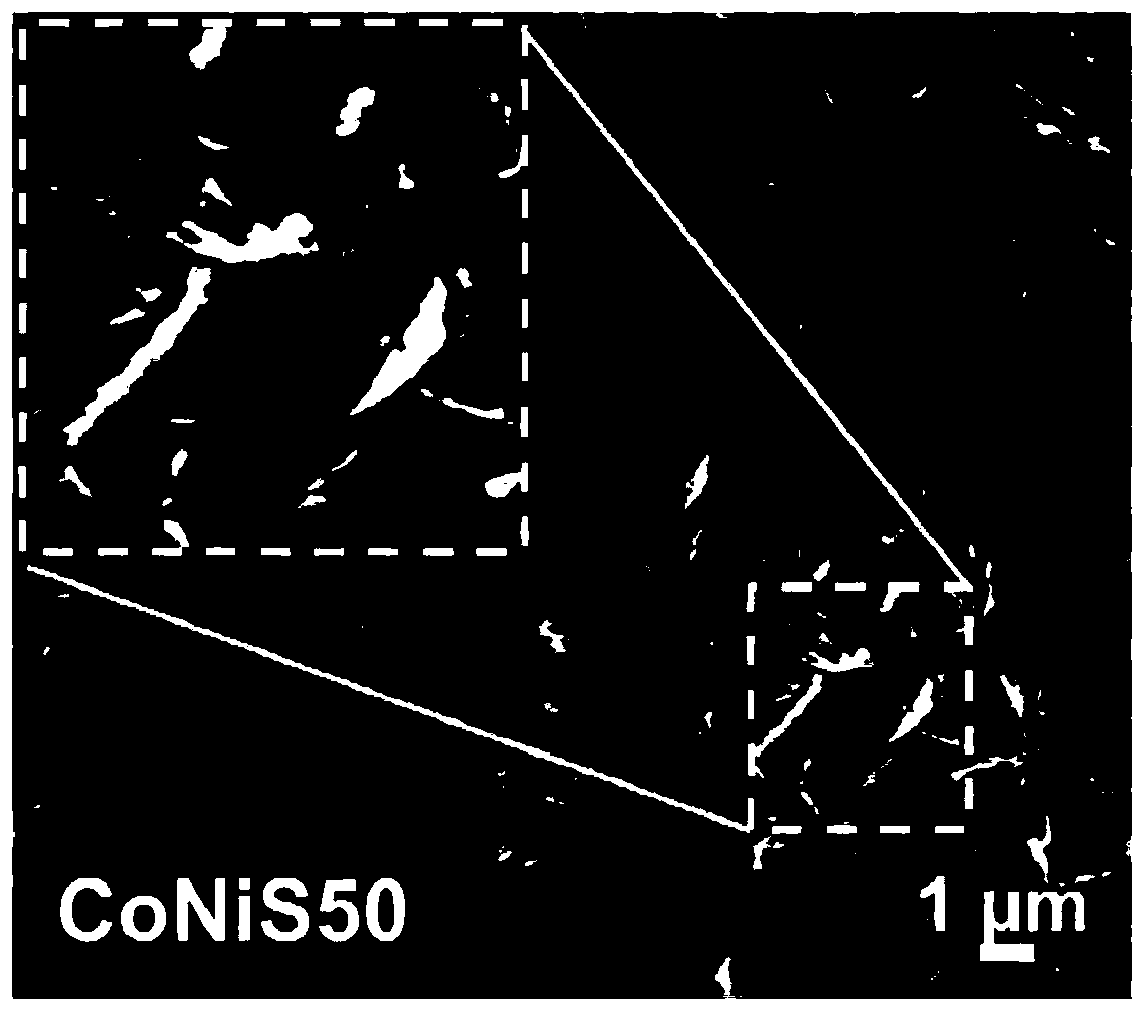
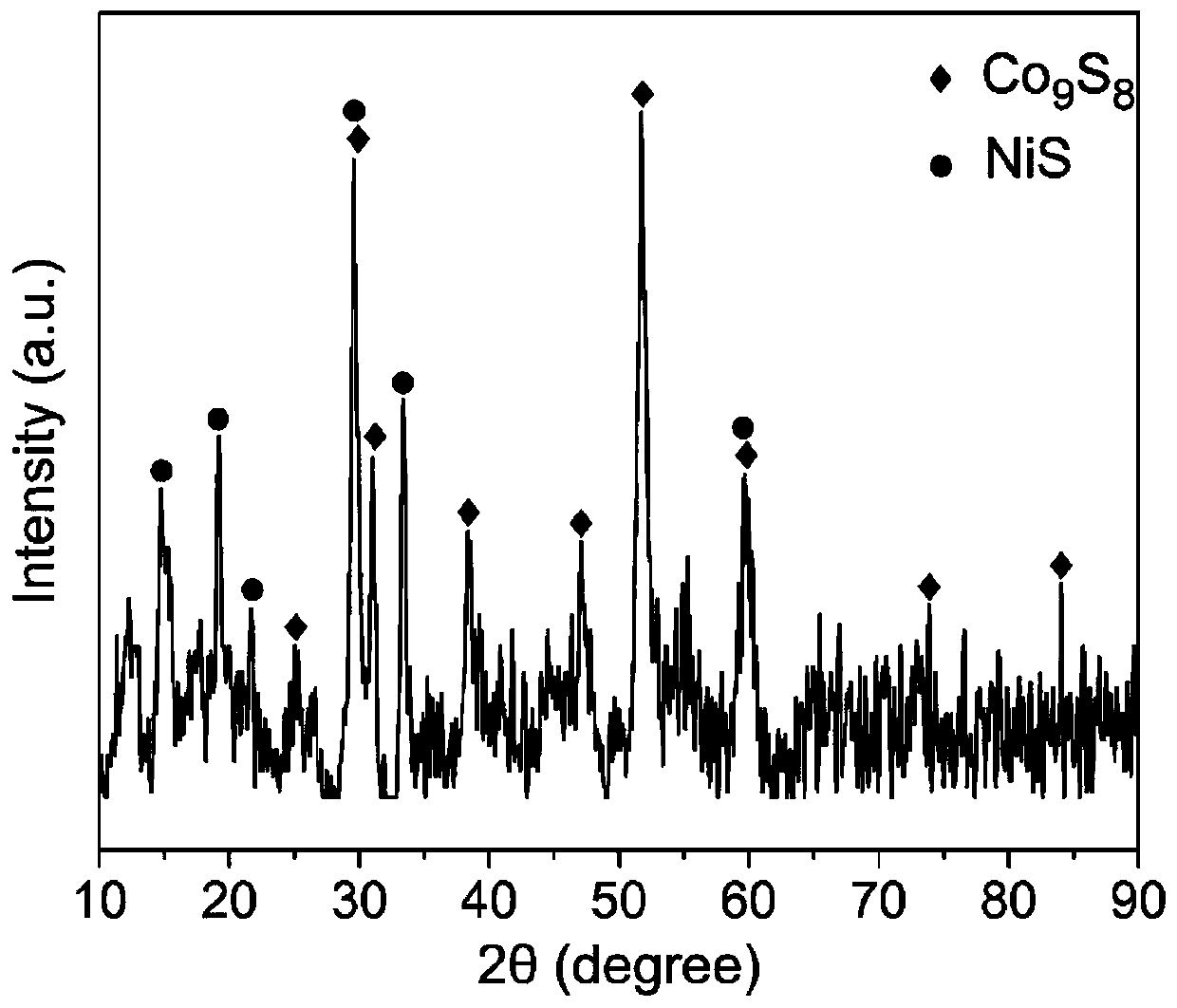
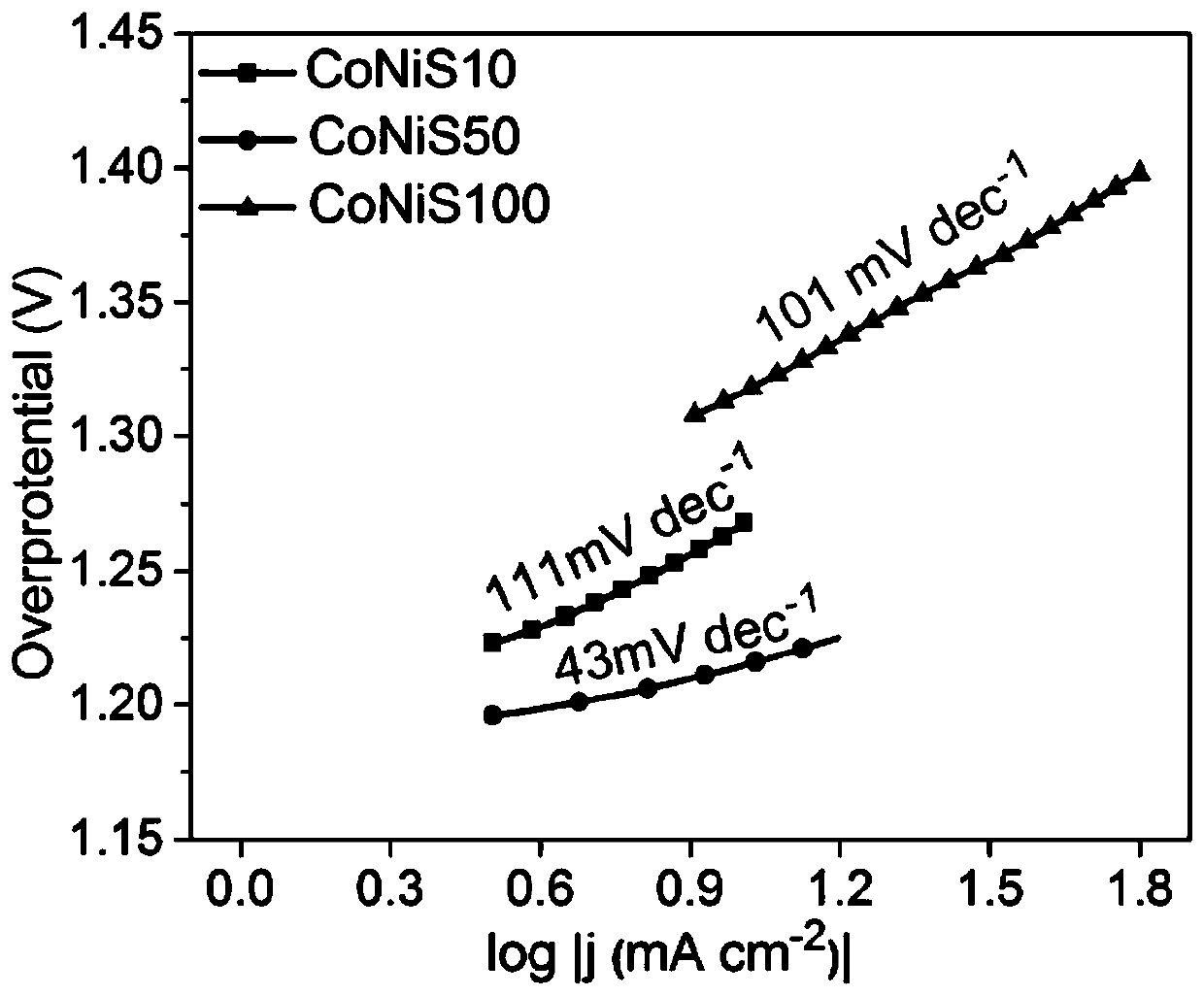
Bimetallic sulfide composite electrocatalyst and preparation method and application thereof
An electrocatalyst and sulfide technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., to achieve the effects of high hydrophilicity, large specific surface area, and shortened path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of a bimetallic sulfide composite electrocatalyst, comprising the steps of:
[0033] (1) First set the area to 0.25cm 2The foamed nickel (NF) was soaked in acetone for 1 hour to remove impurities, and then treated with 3mol / L hydrochloric acid aqueous solution for 2 hours to remove the surface nickel oxide layer. After the treatment, the foamed nickel (NF) was washed with deionized water and ethanol, and finally placed Dry in a vacuum oven at a temperature of 313K for 6-12 hours to obtain a pretreated nickel foam sample.
[0034] (2) 10mg of thiourea and 383mg of Co(NO 3 )·6H 2 O was dissolved in 36mL of deionized water, stirred into a transparent solution and placed in a 50mL hydrothermal kettle with nickel foam (NF), and reacted for 12h at a temperature of 453K. After the hydrothermal reaction was completed and naturally cooled to room temperature, the reactant Rinse several times with deionized water and absolute ethanol. Finally, the reacted...
Embodiment 2
[0036] A preparation method of a bimetallic sulfide composite electrocatalyst, comprising the steps of:
[0037] (1) First set the area to 0.25cm 2 The foamed nickel (NF) was soaked in acetone for 1 hour to remove impurities, and then treated with 3mol / L hydrochloric acid aqueous solution for 2 hours to remove the surface nickel oxide layer. After the treatment, the foamed nickel (NF) was washed with deionized water and ethanol, and finally placed Dry in a vacuum oven at a temperature of 313K for 6-12 hours to obtain a pretreated nickel foam sample.
[0038] (2) 50mg of thiourea and 383mg of Co(NO 3 )·6H 2 O was dissolved in 36mL of deionized water, stirred into a transparent solution and placed in a 50mL hydrothermal kettle with nickel foam (NF), and reacted for 12h at a temperature of 453K. After the hydrothermal reaction was completed and naturally cooled to room temperature, the reactant Rinse several times with deionized water and absolute ethanol. Finally, the reacte...
Embodiment 3
[0042] A preparation method of a bimetallic sulfide composite electrocatalyst, comprising the steps of:
[0043] (1) First set the area to 0.25cm 2 The foamed nickel (NF) was soaked in acetone for 1 hour to remove impurities, and then treated with 3mol / L hydrochloric acid aqueous solution for 2 hours to remove the surface nickel oxide layer. After the treatment, the foamed nickel (NF) was washed with deionized water and ethanol, and finally placed Dry in a vacuum oven at a temperature of 313K for 6-12 hours to obtain a pretreated nickel foam sample.
[0044] (2) 100mg of thiourea and 383mg of Co(NO 3 )·6H 2 O was dissolved in 36mL of deionized water, stirred into a transparent solution and placed in a 50mL hydrothermal kettle with nickel foam (NF), and reacted for 12h at a temperature of 453K. After the hydrothermal reaction was completed and naturally cooled to room temperature, the reactant Rinse several times with deionized water and absolute ethanol. Finally, the react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com