Quantitative detection kit for insulin-like growth factor binding protein 3
A quantitative detection and growth factor technology, applied in the field of medical detection, can solve the problems of low sensitivity, easy to be affected by the environment, low degree of automation, etc., and achieve the effect of improving sensitivity, improving clinical compliance rate, and improving detection rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 kit of the present invention
[0029] 1. Preparation of magnetic particles coated with IGFBP-3 monoclonal antibody
[0030] Fully mix the magnetic particle stock solution with a particle size of 1.00 to 3 μm and a magnetic content of 40%, take out 30 μl, add 300 μl of phosphate buffer to wash 5 times, and wash for 5 minutes each time. When washing, use a magnet to absorb the magnetic particles Fix and discard the supernatant; after washing, add 100 μl of EDC (carbodiimide) and NHS (succinimide ester) activator dissolved in sodium acetate buffer solution, the concentration of the activator is 20 mg / ml, mix and shake for 1 hour; activate Add 300 μl sodium acetate buffer to wash twice, each time for 5 minutes, then add 0.3 μg / person IGF-binding protein 3 antibody, mix and oscillate to coat for 2 hours, after coating, add 30 μl containing 2 % bovine serum albumin phosphate buffer to block 4 times, and finally use phosphate buffer containing ...
Embodiment 2
[0037] The detection method of embodiment 2 kit of the present invention
[0038] 1. Use correct medical technology to collect whole blood samples, centrifuge (speed: 3500r / min, time: 10min), and extract serum / plasma for testing.
[0039] 2. Add calibrator and sample in sequence to the reaction container (hereinafter referred to as "well"), take 20 μl calibrator / sample + 380 μl sample diluent, mix well, add 10 μL / well for detection.
[0040] 3. Add 20 μL of magnetic particle suspension to each well.
[0041] 4. Add 100 μL of enzyme conjugate to each well.
[0042] 5. After mixing, incubate at 37°C for 15 minutes.
[0043] 6. Wash with cleaning solution 5 times.
[0044] 7. Add 100 μL of chemiluminescence substrate to each well, mix well and detect the luminescence value.
[0045] 8. Calculation of results: This kit recommends using a four-parameter fitting method to establish a calibration curve with the concentration value of the calibrator on the x-axis and the log value...
Embodiment 3
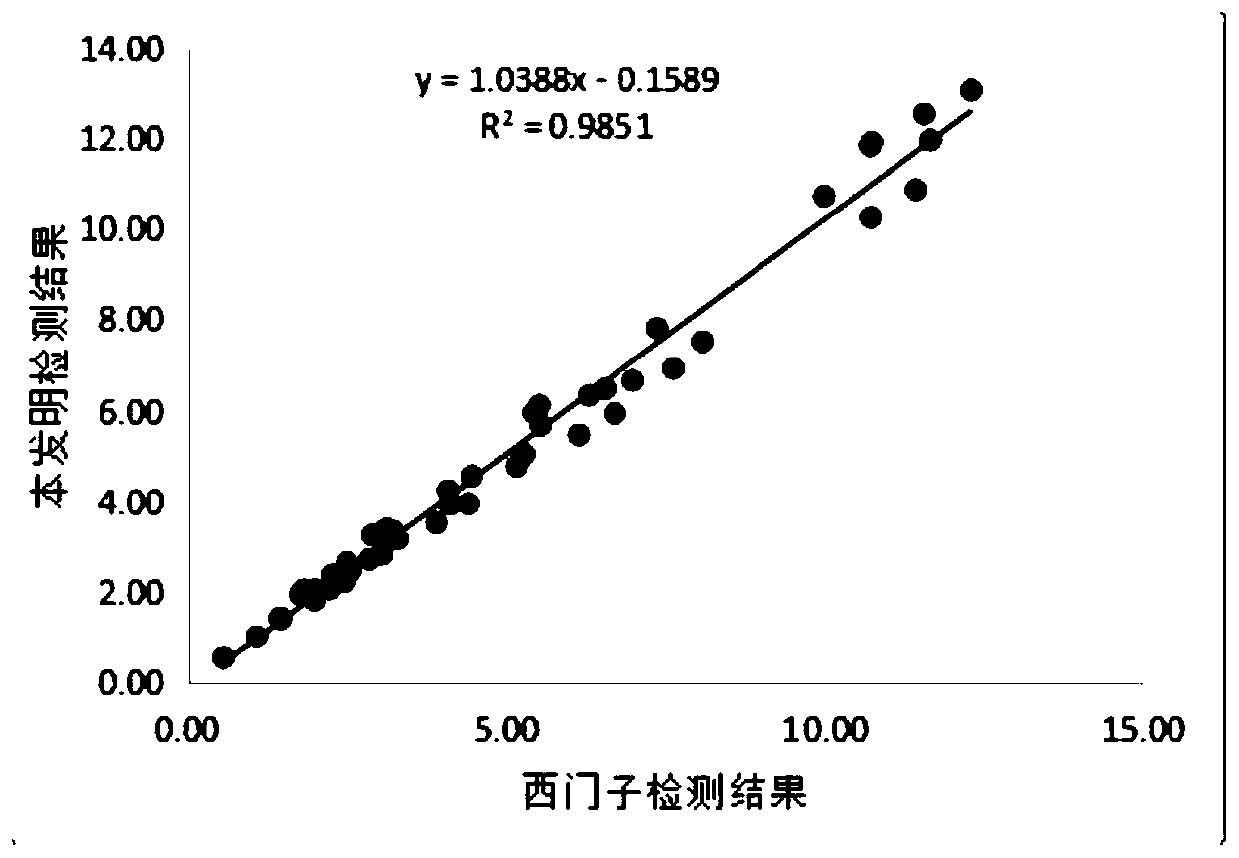
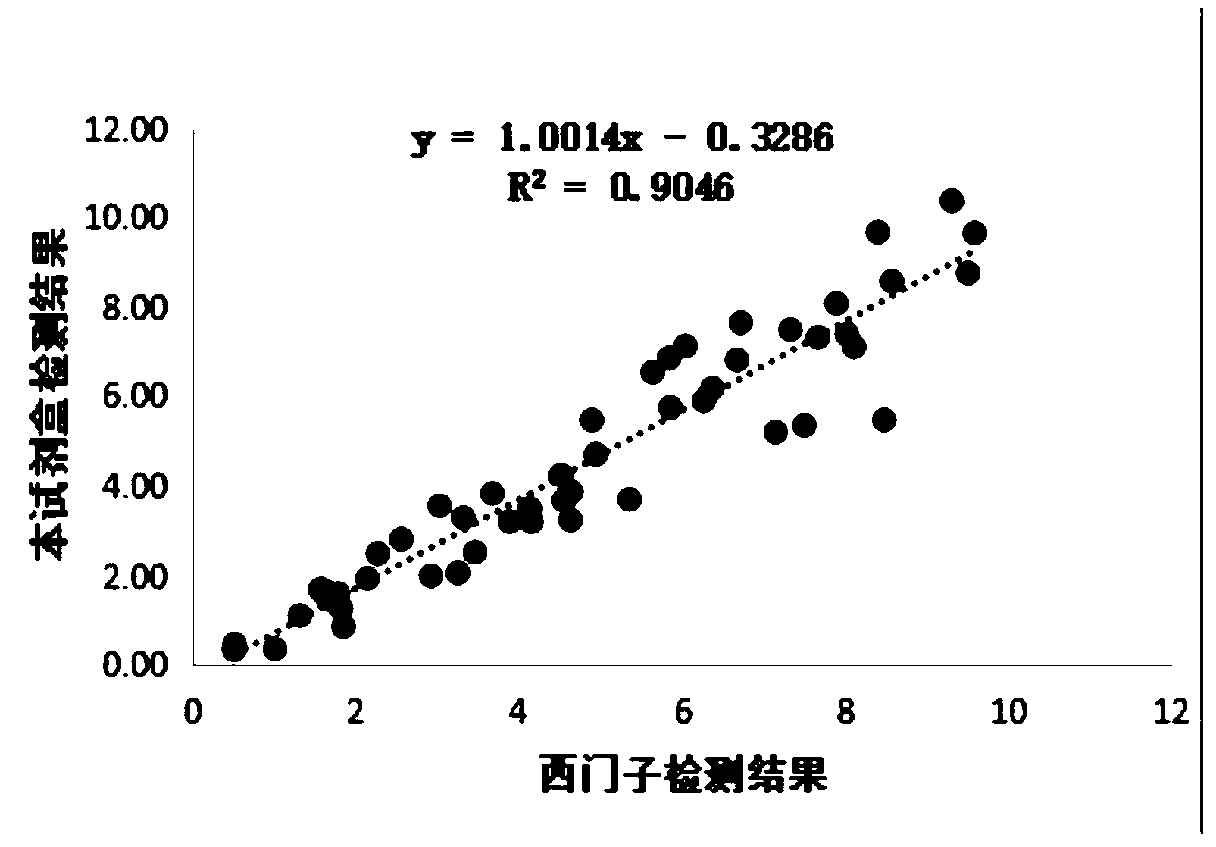
[0046] The performance test of embodiment 3 kit of the present invention
[0047] 1. Analytical sensitivity detection
[0048] According to "EP17A—Protocol for Determining Detection Limits and Quantitative Limits", the analytical sensitivity assessment was carried out, and 5 clinical samples with a value close to 0 were prepared, each sample was repeated 3 times, and a total of 4 days was done, and 60 data with non-negative results were obtained. , if it is less than 60, it is necessary to experiment according to the requirements and make up 60 to calculate the analytical sensitivity.
[0049] Table 1 Sensitivity detection of the kit of the present invention
[0050]
[0051]
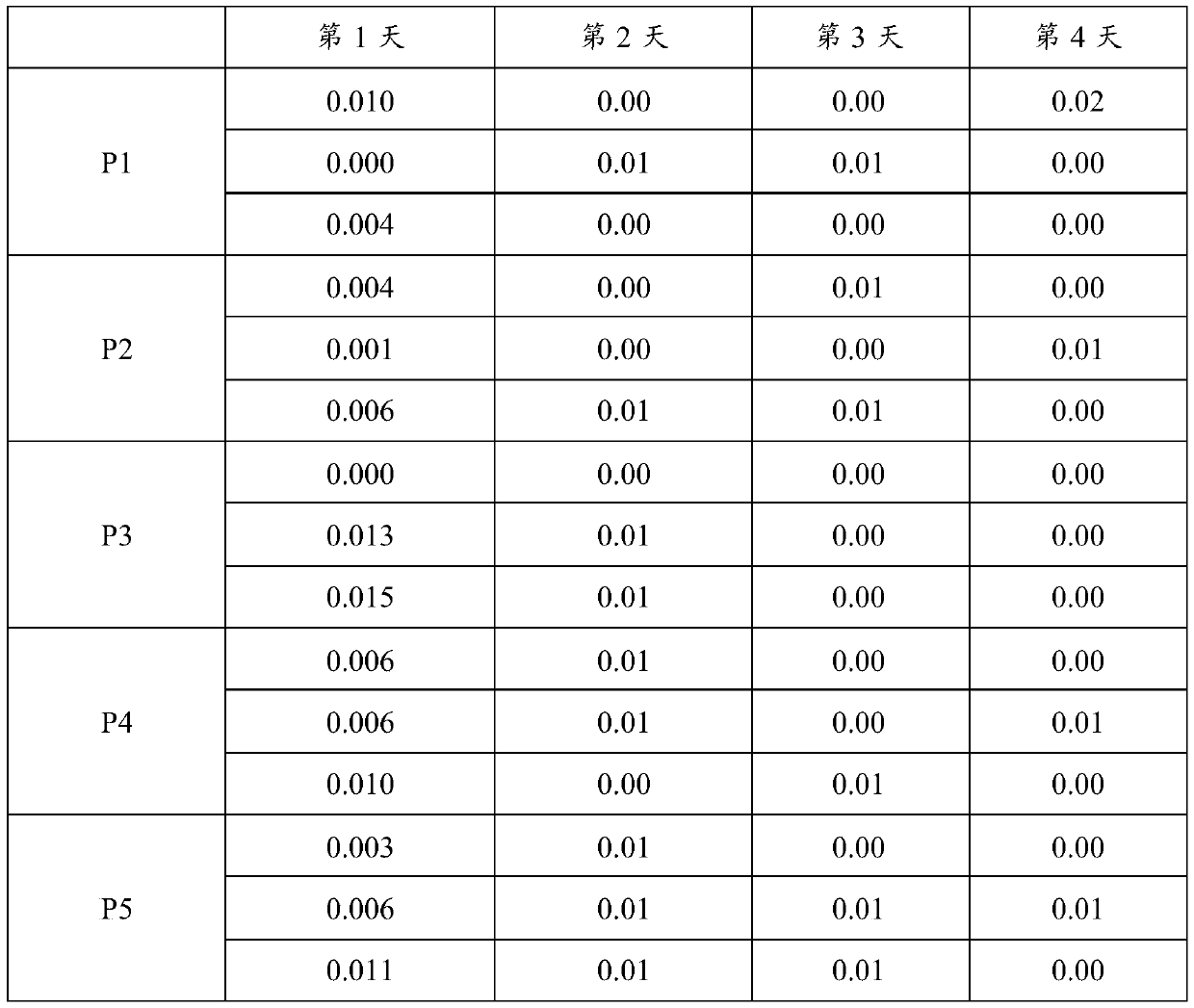
[0052] It can be seen from the results in Table 1 that the analytical sensitivity of the kit of the present invention is 0.02 μg / ml, which is far lower than the 0.1 μg / ml of the existing product, and has higher analytical sensitivity.
[0053] 2. Precision testing
[0054] According to "EP05A2-Pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Analytical sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap