A near-infrared fluorescent probe activated by leucine aminopeptidase and monoamine oxidase, its synthesis method and biological application
A technology of leucine aminopeptidase and monoamine oxidase, applied in the field of fluorescent probes, can solve the problems of low sensitivity and poor specificity of fluorescent probes, and achieve the effects of stable fluorescent signal, high selectivity and good photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
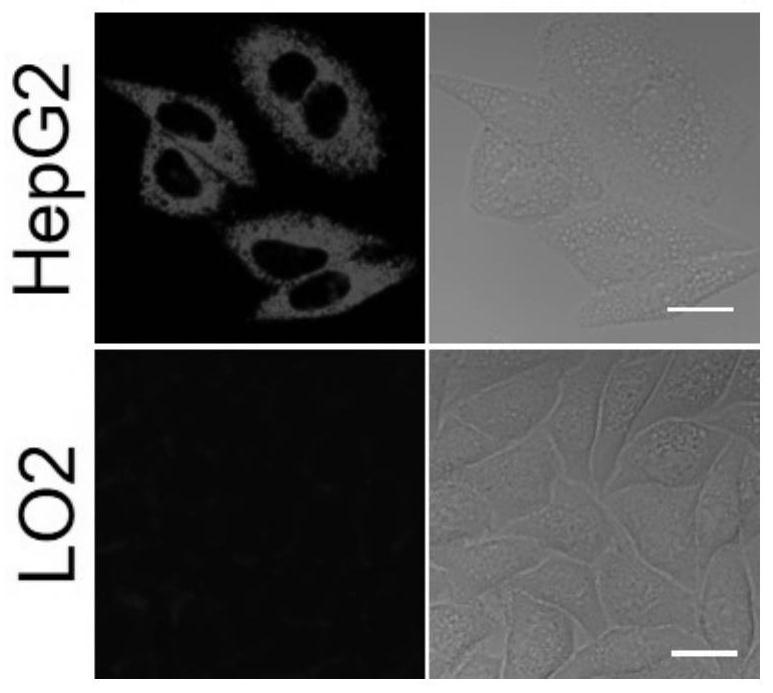
Image
Examples
Embodiment 1
[0047] Example 1 Synthesis of near-infrared leucine aminopeptidase and monoamine oxidase fluorescent probe NML
[0048] (1) Synthesis of Compound 1: p-Hydroxybenzaldehyde (2.44g, 20mmol), (3-bromo-propyl) tert-butyl carbamate (5.95g, 25mmol) and anhydrous potassium carbonate (13.8g, 100mmol) Place in a flask containing acetonitrile (80 mL). And the mixture was stirred at 85°C for 2-3 hours; the solution was cooled and separated by filtration. The filtrate was removed under reduced pressure to give the product as a pale yellow oily liquid (4.81 g, 86% yield) without purification. 1 H NMR (400MHz, CDCl 3 )δ9.79(s,1H),7.74(d,J=8.0Hz,2H),6.92(d,J=8.1Hz,2H),5.07(s,1H),4.04(t,J=5.5Hz, 2H), 3.27(s, 2H), 1.95(d, J=7.1Hz, 2H), 1.37(s, 9H). 13 C NMR (101MHz, CDCl 3 )δ190.73, 163.85, 156.06, 131.90, 129.85, 114.70, 79.07, 66.00, 37.60, 29.46, 28.34;
[0049] (2) Synthesis of Compound 2: Dissolve Compound 1 (2.79g, 10mmol) in trifluoroacetic acid / dichloromethane (20mL / 20mL) at room ...
Embodiment 2
[0058] The detection of embodiment 2NML probe to leucine aminopeptidase and monoamine oxidase in test tube
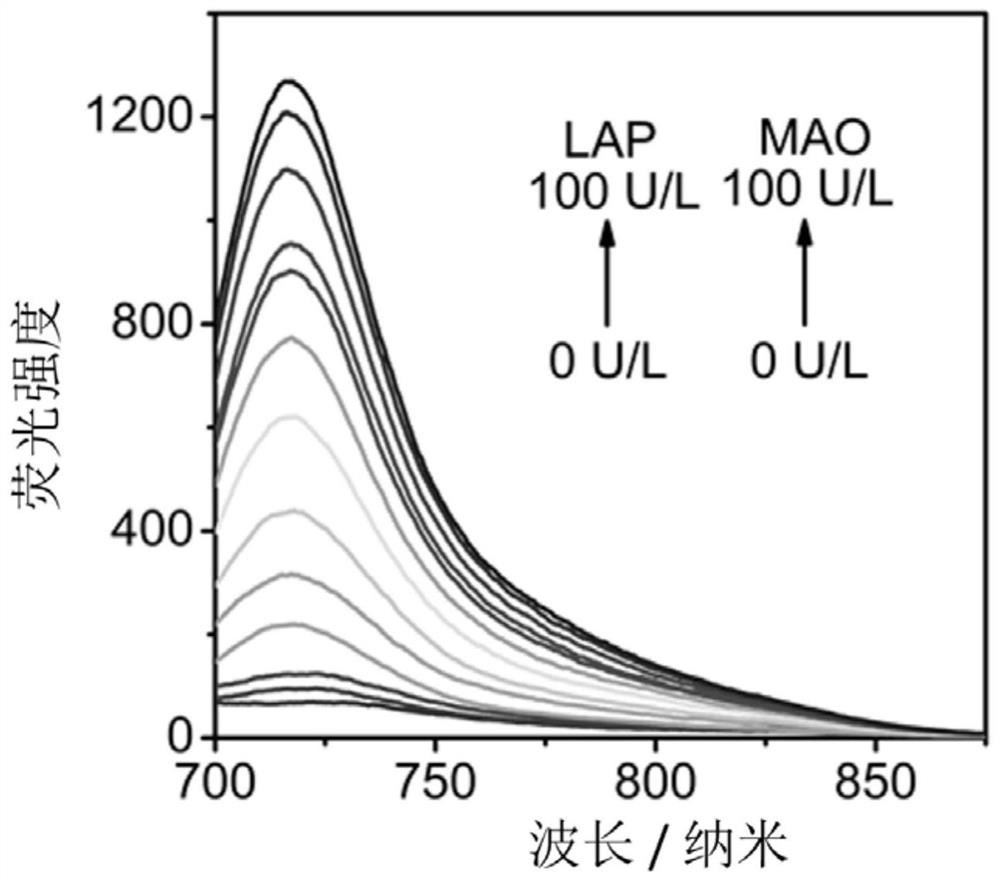
[0059] The NML probe was made into 1 mM DMSO stock solution and stored at -20°C. The detection system is PBS buffer solution (10 mM, pH 7.4, containing 10% DMSO). The reaction system of NML probe and enzyme was shaken at 37° C. for 2 hours, and then its fluorescence emission spectrum was measured. The excitation wavelength of the fluorescence instrument is set to 670nm, and the receiving range of the emission wavelength is 700-900nm. The result is as figure 1 shown, from figure 1 It can be seen that the NML probe responds well to leucine aminopeptidase and monoamine oxidase.
Embodiment 3
[0060] The reaction kinetic investigation of embodiment 3NML probe and enzyme
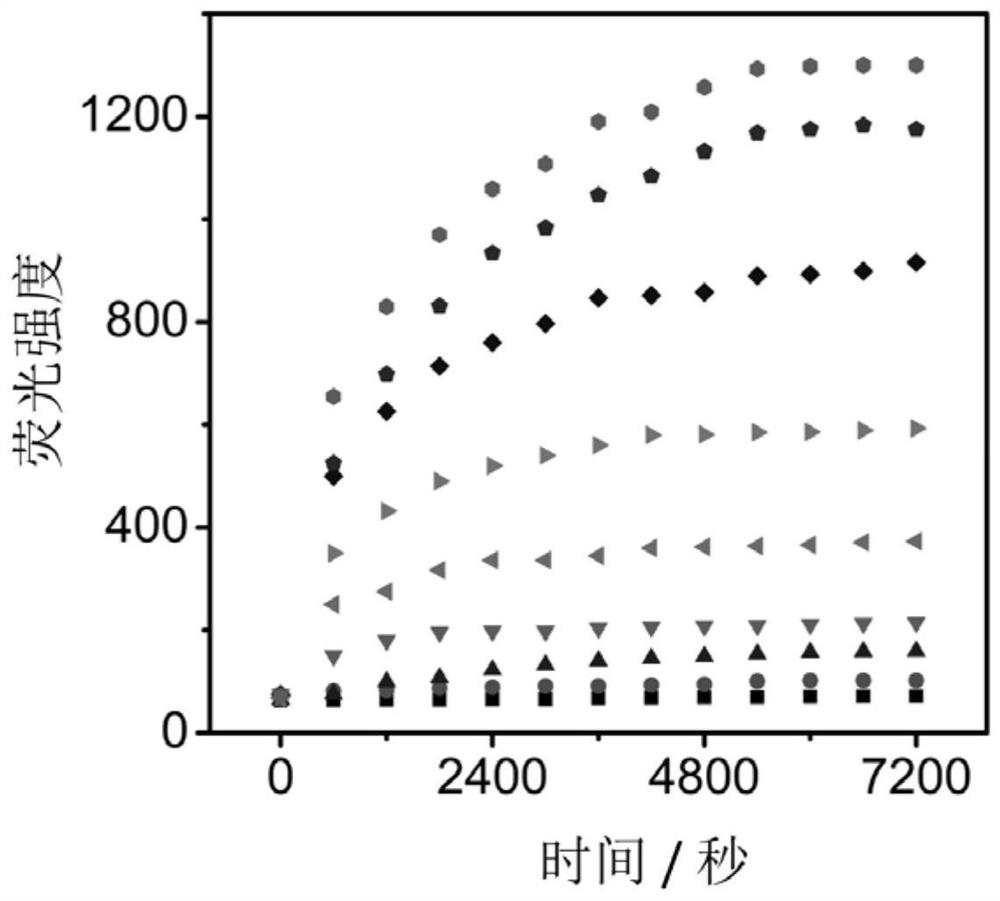
[0061] The reaction system was PBS buffer solution (10 mM, pH 7.4, containing 10% DMSO). The fluorescence emission spectra of NML probes with different activities of leucine aminopeptidase and monoamine oxidase enzymes were measured in real time at 37 °C until the fluorescence intensity no longer changed. Then take the intensity of the maximum emission peak as the ordinate, and the reaction time as the abscissa to make a graph, as figure 2 The reaction kinetic curve shown, from figure 2 As can be seen, the NML probe responds rapidly to both enzymes. Set the excitation wavelength of the fluorometer to 670nm and the emission wavelength to 720nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com