Perylene diimide derivative, preparation method and application of perylene diimide derivative in preparation of ATP (adenosine triphosphate) fluorescent probe
A technology of perylene imide and fluorescent probe, applied in the field of fluorescent probe, can solve the problems of poor solubility of perylene imide derivatives, harsh synthesis conditions, high reaction temperature, etc. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preparation method of perylene imide derivatives, comprising the steps of:
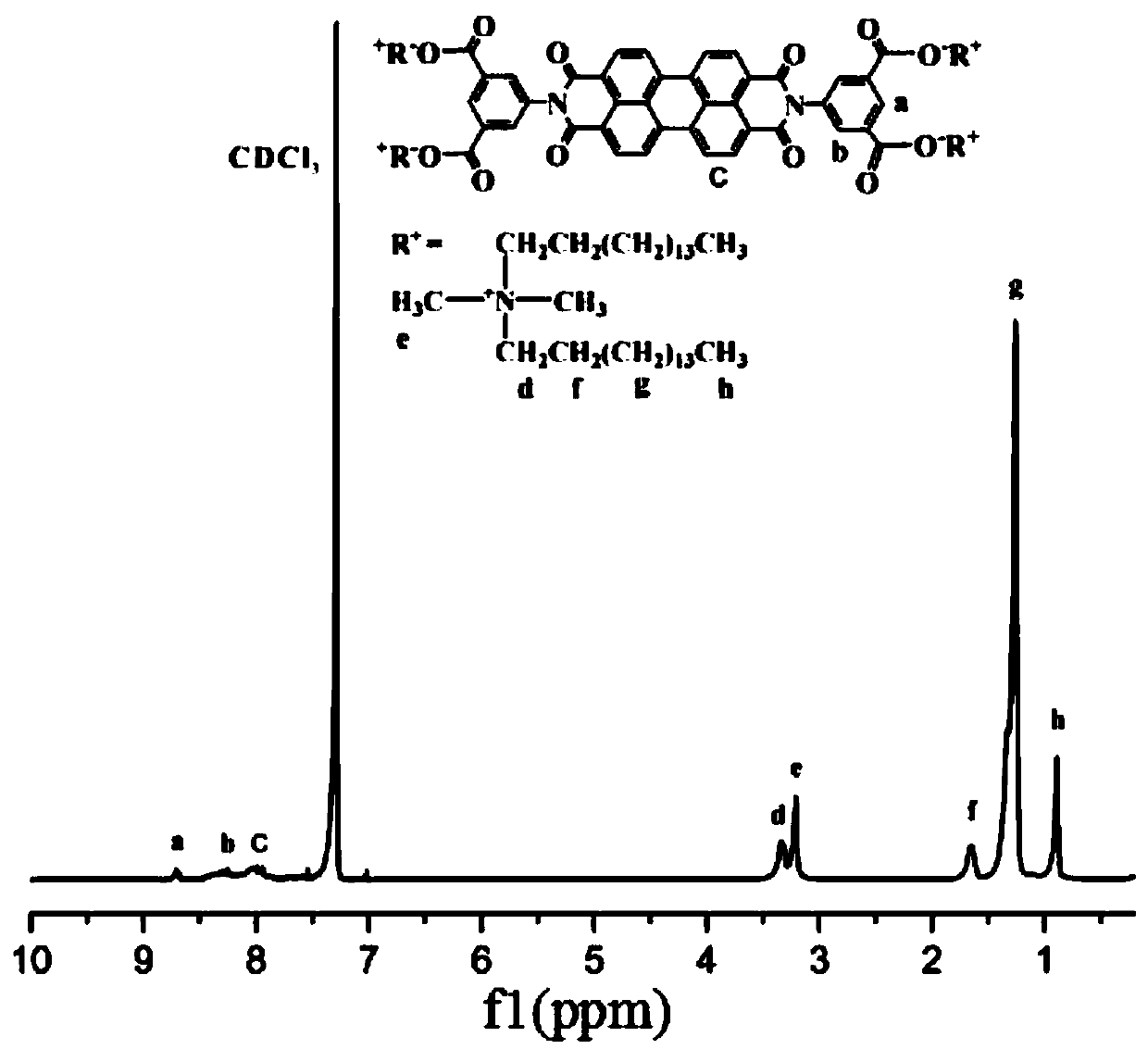
[0040](1) Weigh 1.00g (2.55mmol) 3,4,9,10-perylenetetracarboxylic dianhydride (I), 1.15g (6.37mmol) 5-aminoisophthalic acid, 0.200g (0.912mmol) Zinc acetate in water and 20g imidazole were placed in a round bottom flask, heated to 127°C and stirred for 6 hours under nitrogen protection; after the reaction was completed, transferred to a container with a reflux device, and 100mL of absolute ethanol was added, refluxed 6 hours; stop heating and stirring, leave standstill 10h; Vacuum suction filtration obtains dark red precipitate, and washes repeatedly 3 times with absolute ethanol, obtains compound (II), and the productive rate is 83.2%, and its structure is passed by nuclear magnetic spectrum and Infrared for confirmation; hydrogen spectrum: δ (TMS, ppm): 7.15 (m, 8H), 8.11 (s, 4H), 8.43 (s, 2H); infrared (KBr, cm -1 ):1698,1664,1596,1353,1251;
[0041] (2) Weigh 33.7mg (0.6mmol) of potassi...
Embodiment 2
[0050] A kind of purposes that perylene imide derivative prepares ATP fluorescent probe, comprises the steps:
[0051] Put the peryleneimide derivative (IV) prepared in Example 1 into an aqueous ethanol solution with a volume concentration of 50%, shake it, and prepare a kind of ATP fluorescent probe; the peryleneimide derivative (IV) and ethanol The molar ratio is 1:12500.
Embodiment 3
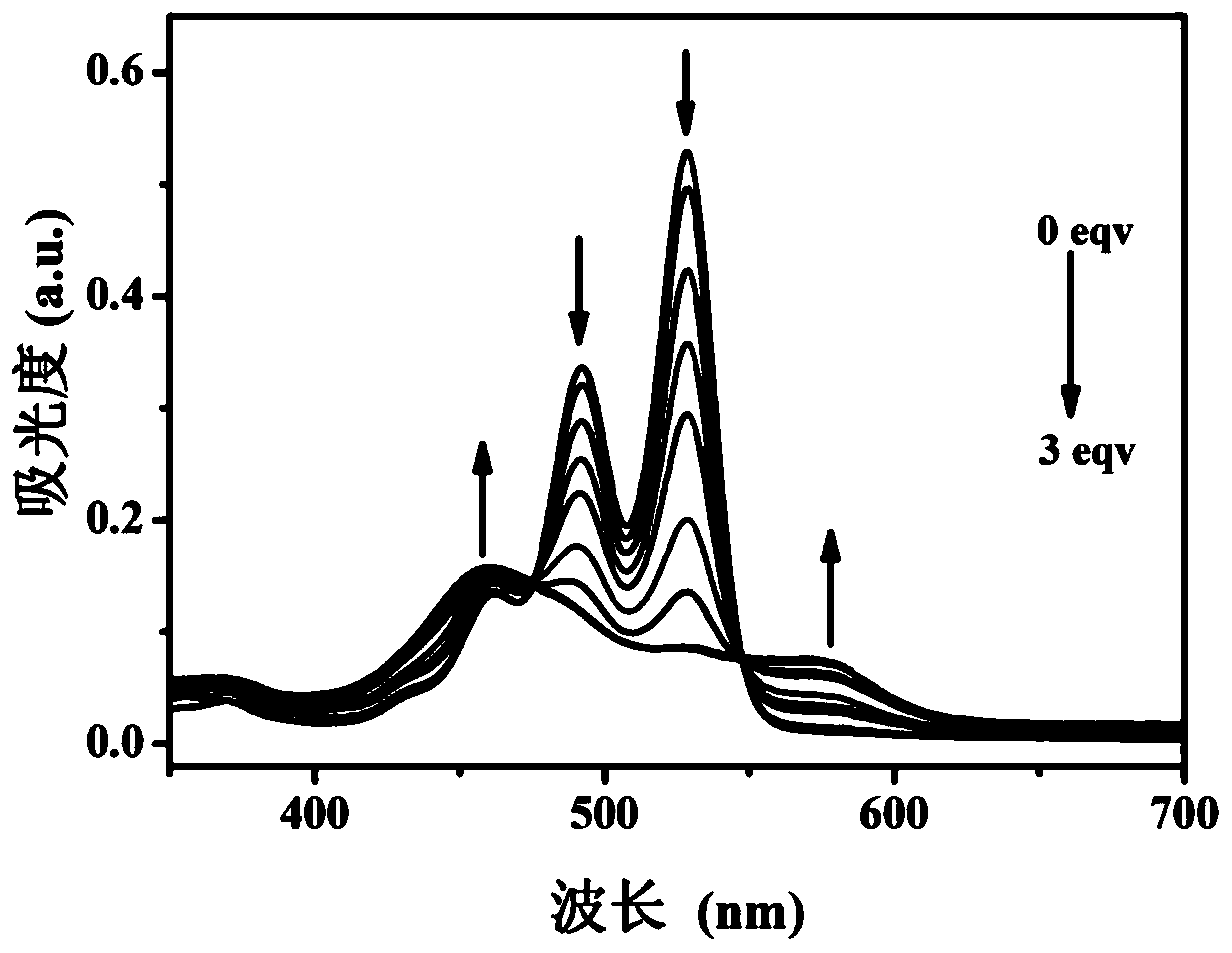
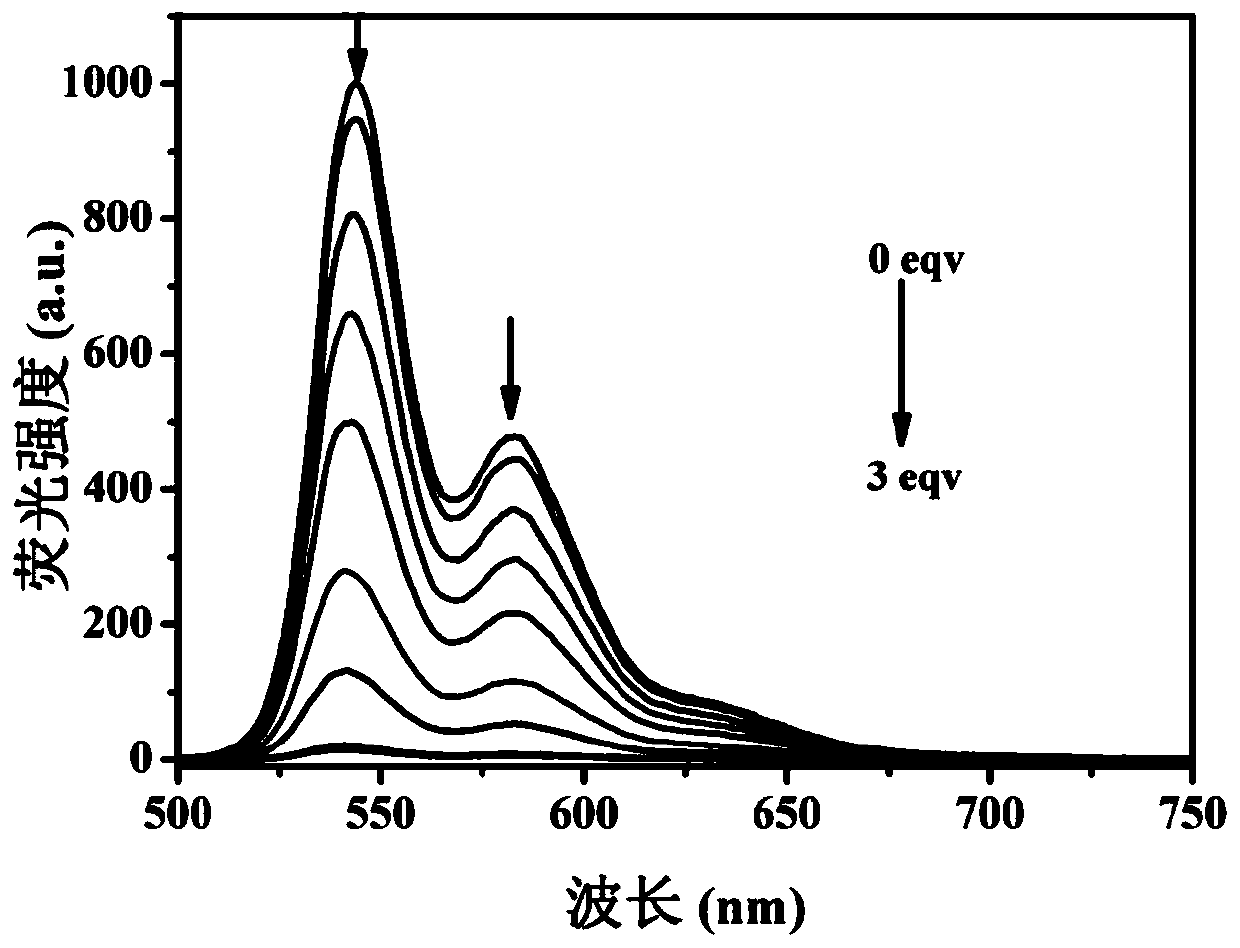
[0053] The measurement of the optical properties of the ATP fluorescent probe prepared in embodiment 2 under different ATP concentrations
[0054] Get 9 parts of ATP fluorescent probe solution (I) that the volume is 5mL prepared by Example 2, then add respectively 5mL containing 0, 0.4, 0.8, 1.2, 1.6, 2, 2.4, 2.8, 3 equivalents The ethanol aqueous solution of the ATP (the ethanol aqueous solution volume concentration is 50%), measure its ultraviolet-absorption spectrum and fluorescence spectrum, see figure 2 and image 3 . From figure 2 It can be seen from the UV-absorption spectrum that without adding ATP, the maximum absorption wavelength is around 529nm, and there are two peaks around 492nm and 460nm. With the gradual addition of ATP, the peaks at 529 and 492nm decreased gradually, while the absorption peak at 460nm increased gradually, a new peak appeared at 577nm, and a clear isosbestic point appeared at 548nm. exist image 3 It can be seen from the fluorescence sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com