New application of sesquiterpenoids
A compound and solvate technology, applied in the field of pharmaceutical use, can solve the problems of unrecorded inflammation treatment effects, etc., and achieve good inflammation inhibition, good inflammation, and the effect of inhibiting nuclear translocation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This embodiment provides a compound shown in formula (1), coded as LB, that is, hereinafter LB all represent compounds shown in formula (1)
[0040]
[0041] Its preparation method is as follows:
[0042] (1) Weigh 700g of Herba chinensis medicinal material powder, add 7L of ethanol with 50% concentration, heat to a slight boil, reflux for extraction for 1 hour, filter after cooling, collect the filtrate, and use 50% ethanol for reflux to extract the filter residue according to the above method 2 Second-rate. Combine the three extracts and concentrate under reduced pressure to 700mL;
[0043] (2) Weigh 700 g of activated D101 macroporous resin, add it to the above concentrated solution, let it stand for adsorption for 24 hours, and then load it into a separation column. Elute with pure water until the eluent is nearly colorless, then elute with appropriate amount of 50% and 95% ethanol in turn until the eluent is nearly colorless, combine the eluents, concentrate u...
experiment example 1
[0047] To detect the toxicity of LB to RAW264.7 cells
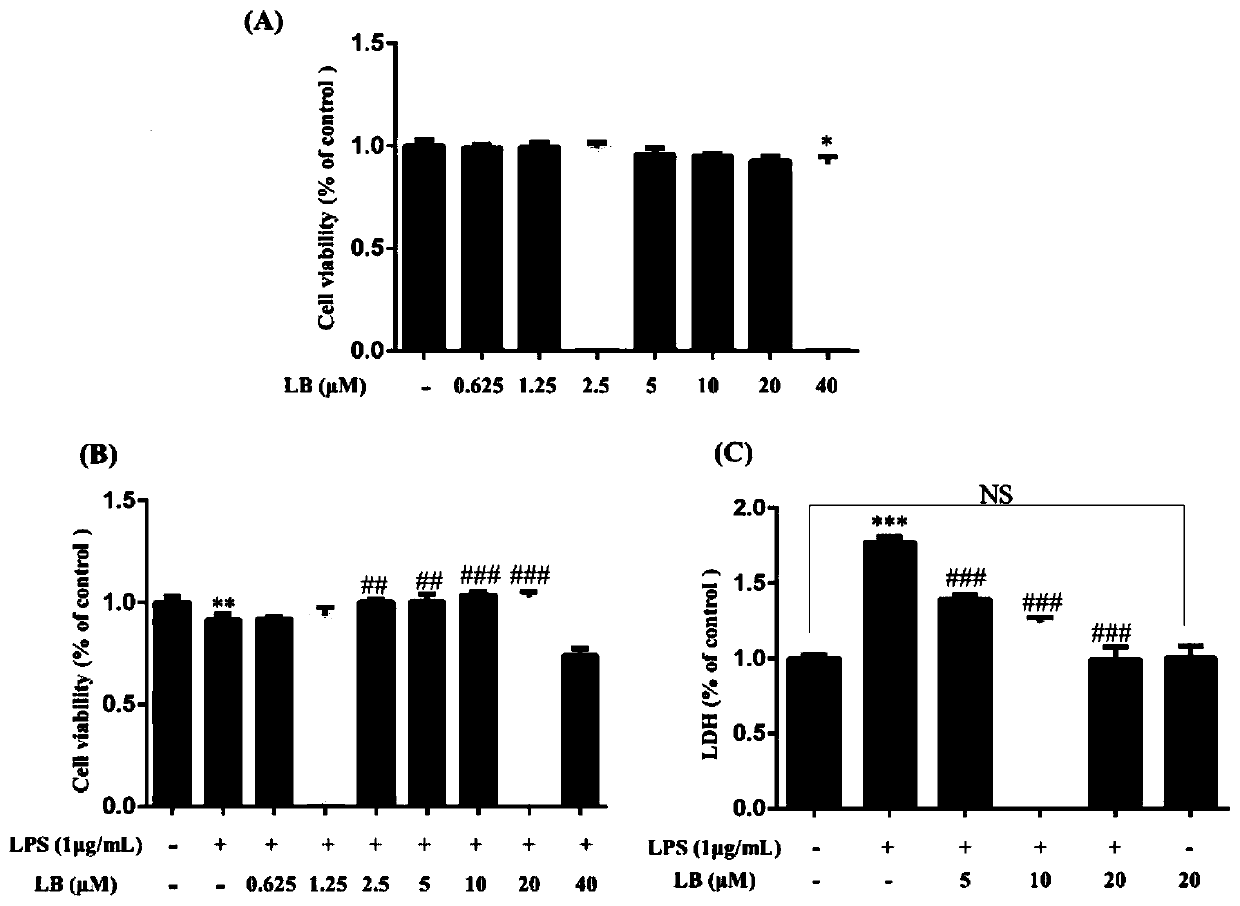
[0048] Specific detection method: RAW264.7 was treated with LB (0.625 μM, 1.25 μM, 2.5 μM, 5 μM, 10 μM, 20 μM and 40 μM) without adding lipopolysaccharide (LPS) and adding LPS (1 μg / mL), respectively Cells were left for 24 hours before cell viability was measured by MTT assay. For test results, see figure 1 A and B in,figure 1 A in A is the MTT detection result of different concentrations of LB when no LPS is added; figure 1 B in B is the MTT detection results of different concentrations of LB when the LPS is 1 μg / mL; the data are the mean ± SD of the smallest three independent experiments.
[0049] RAW264.7 cells were treated with LB (5 μM, 10 μM, and 20 μM) for 24 hours without adding lipopolysaccharide (LPS) and adding LPS (1 μg / mL), and then measured by LDH assay kit Lactate dehydrogenase (LDH) content. For test results, see figure 1 In C, data are mean ± SD of a minimum of three independent experiments.
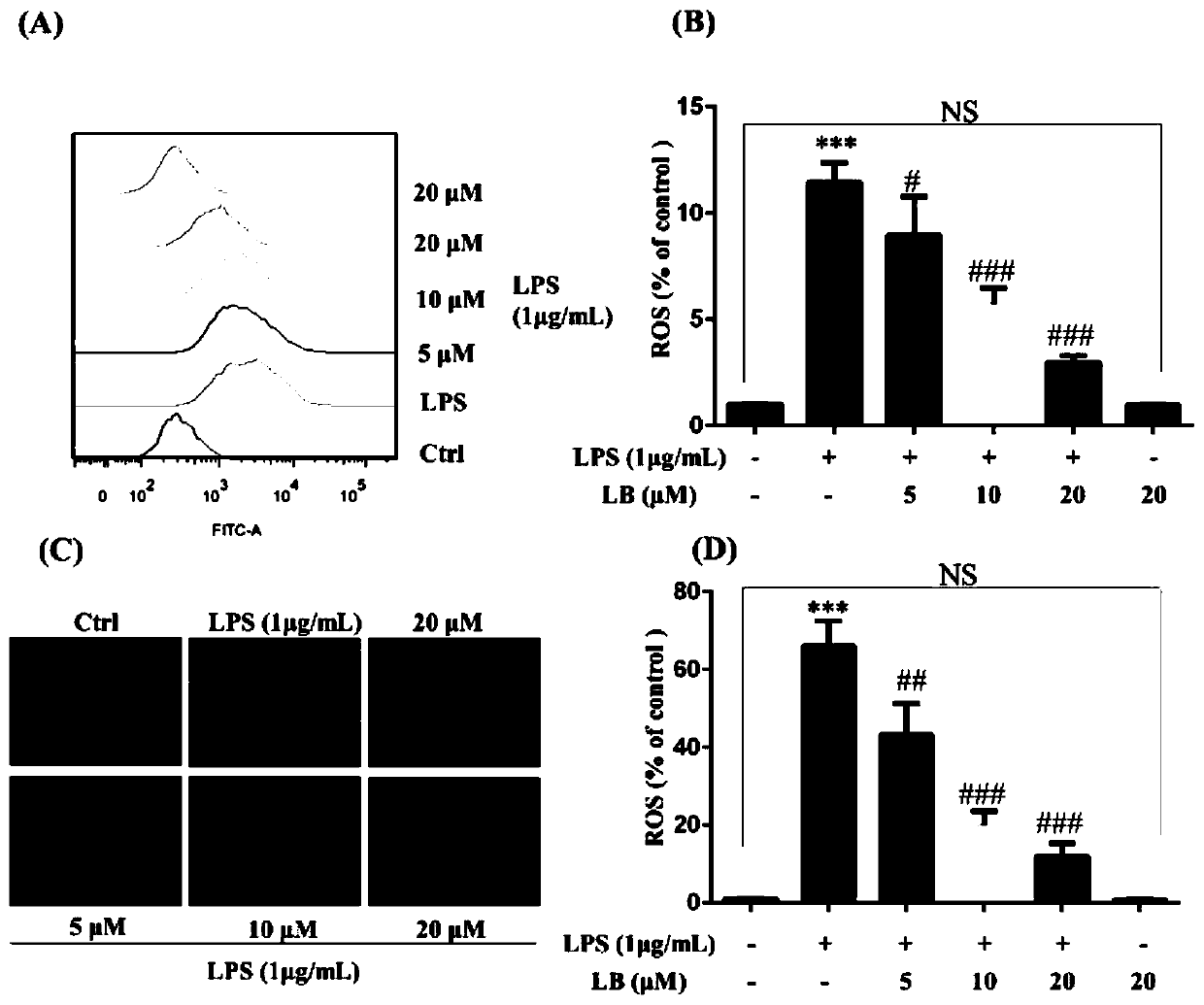
[005...
experiment example 2
[0052] Detection of LB on NO, PGE in RAW264.7 cells 2 and pro-inflammatory proteins
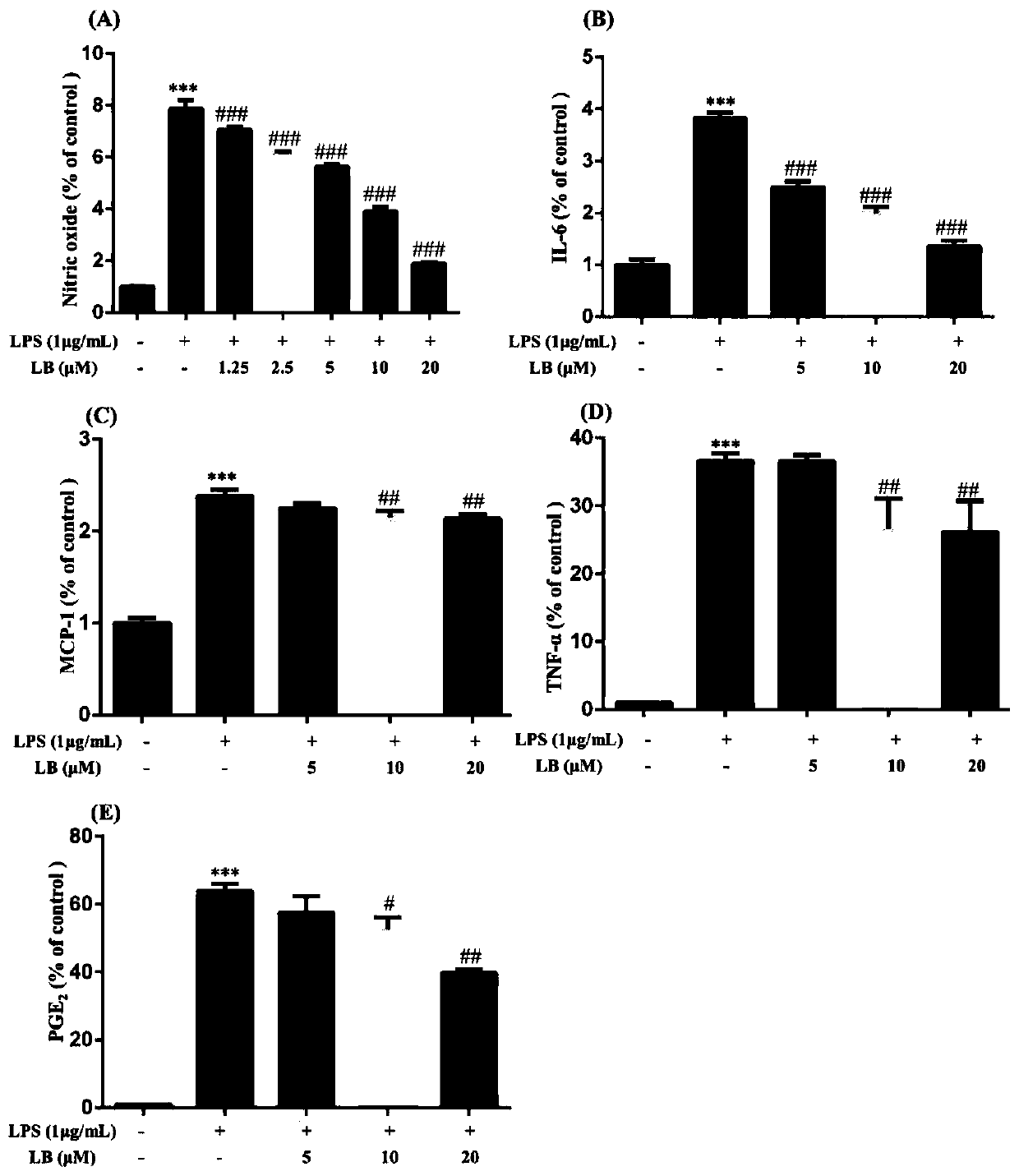
[0053] Specific detection method: RAW264.7 cells were pretreated with LB (5 μM, 10 μM, 20 μM) for 1 hour, and then stimulated with LPS (1 μg / mL) for 12 hours, collected supernatant, and detected NO with Griess reagent, ELISA kit Detection of IL-6, MCP-1, TNF-α and PGE 2 . For test results, see figure 2 (A)-(E) in, where figure 2 A in is the test result of No, figure 2 B in is the detection result of IL-6, figure 2 C in is the detection result of MCP-1, figure 2 D in is the detection result of TNF-α, figure 2 E in is PGE 2 test results. Data are means ± SD of a minimum of three independent experiments.
[0054] according to figure 2 It can be seen that compared with the control, ***p2 and production of proinflammatory proteins.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com