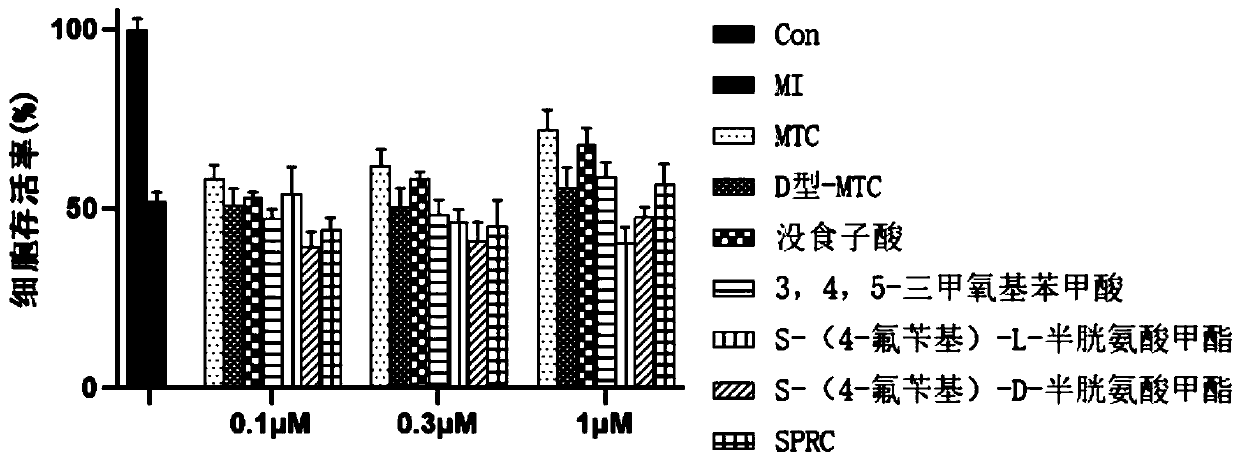
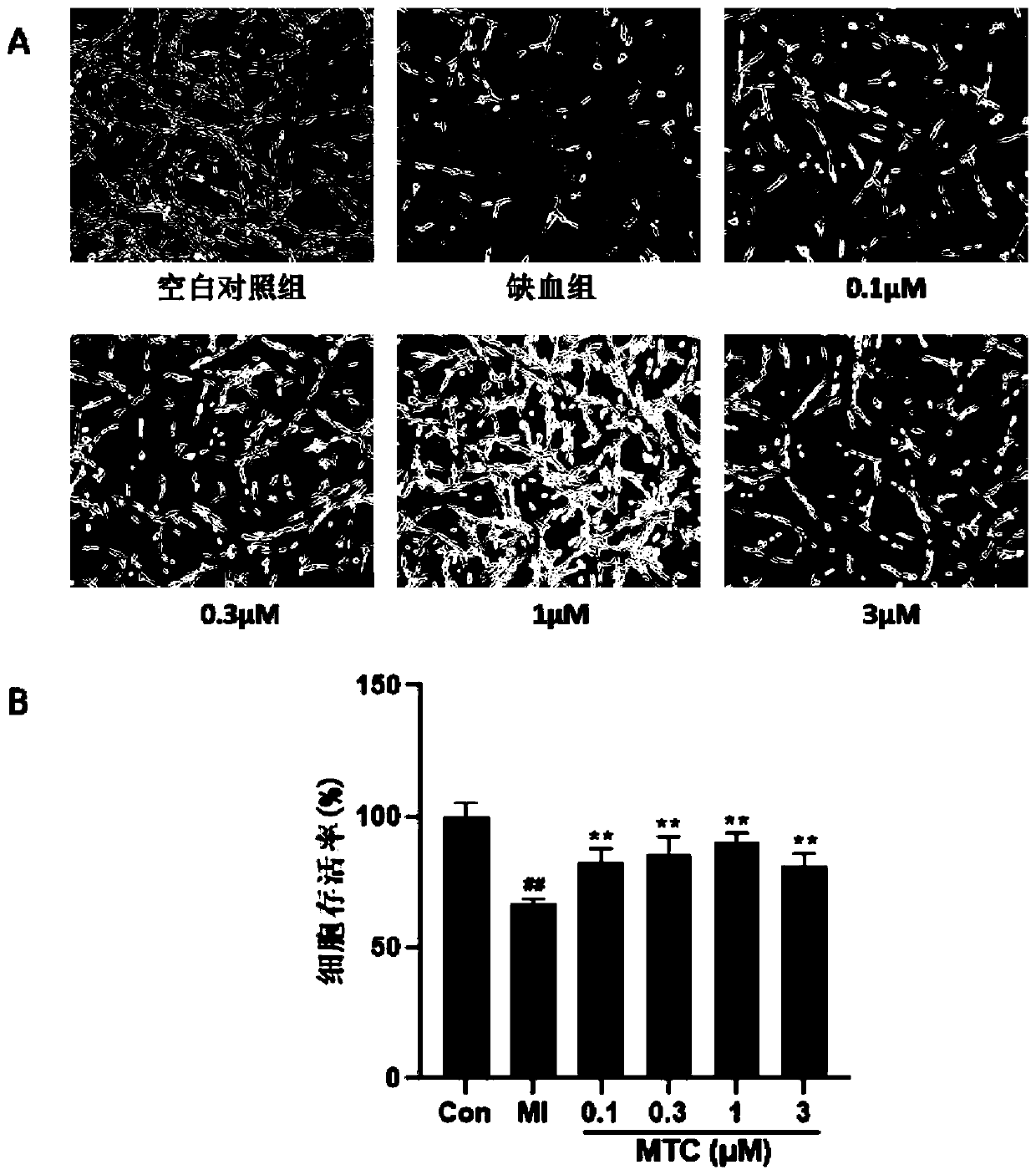
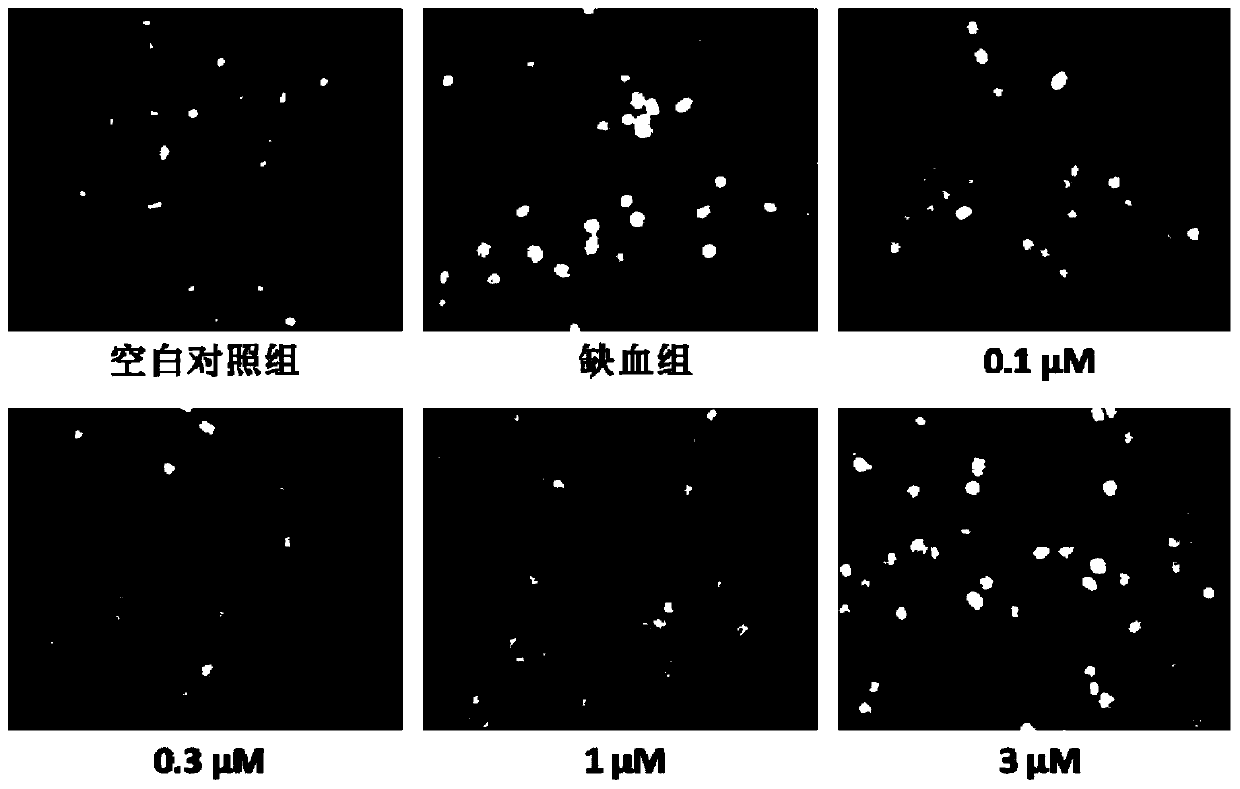
Hydrogen sulfide donor, preparation method and applications thereof
A hydrogen sulfide and donor technology, applied in the field of medicine, can solve the problems of easy oxidative deterioration, volatile, uncontrollable dosage, etc., and achieve the effects of reducing endoplasmic reticulum stress, increasing survival rate, and activating signaling pathways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The present embodiment is the donor (MTC) of hydrogen sulfide according to the present invention, and its structural formula is as shown in formula (I):
[0051]
[0052] The preparation method of the donor (MTC) of above-mentioned hydrogen sulfide, comprises the steps:
[0053] (1) Dissolve 1mmol of the compound shown in formula (II) in 40mmol of thionyl chloride (SOCl 2 ), reflux the reaction (rf) at 70°C until complete, track the reaction to the end by thin-layer chromatography, spin down the solvent under reduced pressure, and obtain the compound shown in the intermediate product formula (Ⅲ);
[0054] (2) Dissolve 1mmol of the compound represented by formula (III) described in step (1) in 45mmol of dichloromethane (DCM), then add 2mmol of triethylamine (TEA) and stir the reaction for 10min under ice-bath conditions, then add 1mmol of the compound shown in formula (Ⅳ), continue to stir until the end of the reaction, spin the solvent to dryness under reduced press...
Embodiment 2
[0060] The present embodiment is the donor (MTC) of hydrogen sulfide according to the present invention, and its structural formula is as shown in formula (I):
[0061]
[0062] The preparation method of the donor (MTC) of above-mentioned hydrogen sulfide, comprises the steps:
[0063] (1) Dissolve 1 mmol of the compound shown in formula (II) in 45 mmol of thionyl chloride (SOCl 2 ), reflux the reaction (rf) at 80°C until complete, track the reaction to the end by thin-layer chromatography, spin the solvent under reduced pressure, and obtain the compound shown in the intermediate product formula (Ⅲ);
[0064] (2) Dissolve 1mmol of the compound shown in formula (III) described in step (1) in 50mmol of dichloromethane (DCM), then add 3mmol of triethylamine (TEA) and stir the reaction for 30min under ice-bath conditions, then add 1mmol of the compound shown in formula (Ⅳ), continue to stir until the end of the reaction, spin the solvent to dryness under reduced pressure, and ...
Embodiment 3
[0068] The present embodiment is the donor (MTC) of hydrogen sulfide according to the present invention, and its structural formula is as shown in formula (I):
[0069]
[0070] The preparation method of the donor (MTC) of above-mentioned hydrogen sulfide, comprises the steps:
[0071] (1) Dissolve 1 mmol of the compound shown in formula (II) in 43 mmol of thionyl chloride (SOCl 2 ), reflux the reaction (rf) at 75°C until complete, track the reaction to the end by thin-layer chromatography, spin the solvent under reduced pressure, and obtain the compound shown in the intermediate product formula (Ⅲ);
[0072] (2) Dissolve 1mmol of the compound shown in formula (III) described in step (1) in 48mmol of dichloromethane (DCM), then add 2.5mmol of triethylamine (TEA) and stir the reaction for 20min under ice-bath conditions, then Add the compound represented by the formula 1mmol (IV), continue to stir until the reaction is completed, spin the solvent under reduced pressure, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com