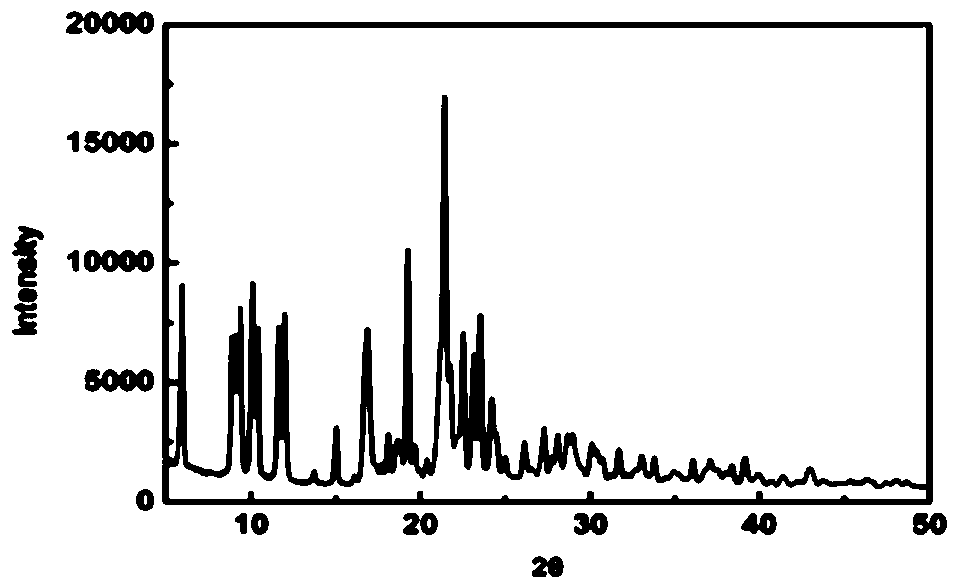
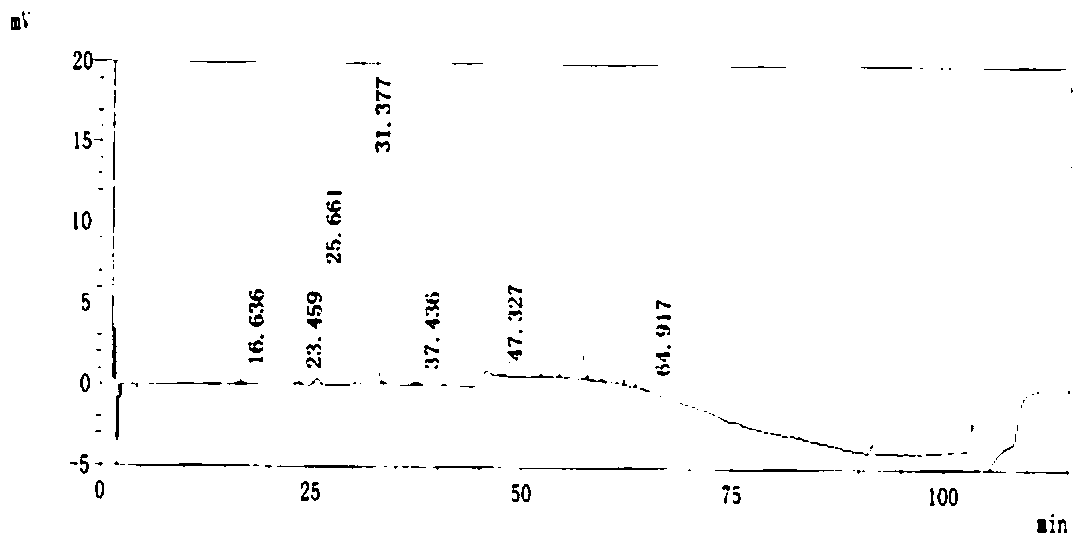
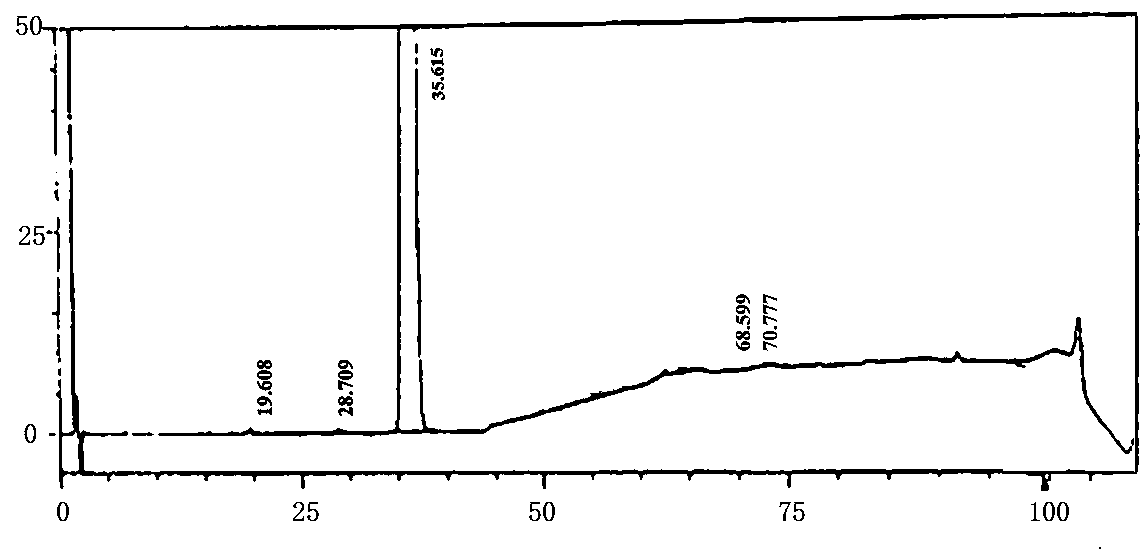
A kind of preparation method of i crystal form atorvastatin calcium
A technology of atorvastatin calcium and atorvastatin, which is applied in organic chemistry methods, organic chemistry and other directions, and can solve the problems of crystal form and purity of atorvastatin calcium.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present invention provides a preparation method of I crystal form atorvastatin calcium, which comprises the following steps:
[0036] S1. Preparation of atorvastatin ester
[0037] Atorvastatin intermediate L1, (4R, 6R)-2-[6-[2-[2-(4-fluorophenyl)-5-isopropyl ester-3-phenyl-4-(phenylamine Formyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]tert-butyl acetate (hereinafter referred to as "Intermediate L1"), purity testing The main peak content should not be less than 98%, the single impurities should not be higher than 0.3%, and the total impurities should not be higher than 1.0%. Under acidic conditions, the isopropyl group protection should be removed. The reaction process is as follows:
[0038]
[0039] In some specific embodiments of the present invention, the atorvastatin intermediate L1 is dissolved in alcohol, and the alcohol is selected from one or more of methanol, ethanol, n-propanol, or isopropanol, preferably methanol.
[0040] In some specific embodiments of...
Embodiment 1
[0063] Put 120g of Intermediate L1 and 600ml of methanol into a 2000ml reaction kettle, then add the prepared hydrochloric acid solution (142g purified water, 16.4g hydrochloric acid), slowly increase the temperature to 30°C, react for 5 hours, TLC panel monitoring, raw material point The disappearance is the end of the reaction.
[0064] After the reaction in the previous step is complete, add 10% NaOH solution (sodium hydroxide 14.2g, water 127.8g) to the reaction solution, react at 30°C for 5 hours, monitor by TLC dot panel, and the atorvastatin ester point disappears. end.
[0065] When the reaction in the previous step is complete, directly add calcium acetate aqueous solution (calcium acetate 17.8g; water 240g), stir the reaction at 20°C for 2 hours, and centrifuge to obtain the crude atorvastatin calcium wet product. Dry in a circulating hot air oven at 50°C to obtain crude atorvastatin calcium as a dry product.
[0066] Add the crude atorvastatin calcium dry product to the ...
Embodiment 2
[0077] Put 120g of Intermediate L1 and 600ml of methanol into a 2000ml reaction kettle, then add the prepared hydrochloric acid solution (142g purified water, 16.4g hydrochloric acid), slowly increase the temperature to 30°C, react for 5 hours, TLC panel monitoring, raw material point The disappearance is the end of the reaction.
[0078] After the reaction in the previous step is complete, add 10% NaOH solution (sodium hydroxide 14.2g, water 127.8g) to the reaction solution, react at 30°C for 5 hours, monitor by TLC dot panel, and the atorvastatin ester point disappears. end.
[0079] When the reaction in the previous step is complete, directly add calcium acetate aqueous solution (calcium acetate 17.8g; water 240g), stir the reaction at 20°C for 2 hours, and centrifuge to obtain the crude atorvastatin calcium wet product. Dry in a circulating hot air oven at 50°C to obtain crude atorvastatin calcium as a dry product.
[0080] Add the crude atorvastatin calcium dry product to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com