Halogenated aromatic ketone derivative based organic electroluminescent material, preparation method therefor and application of organic electroluminescent material
A technology of electroluminescent materials and derivatives, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of efficiency roll-off, and achieve the effect of efficiency roll-off, high device efficiency, and strong applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
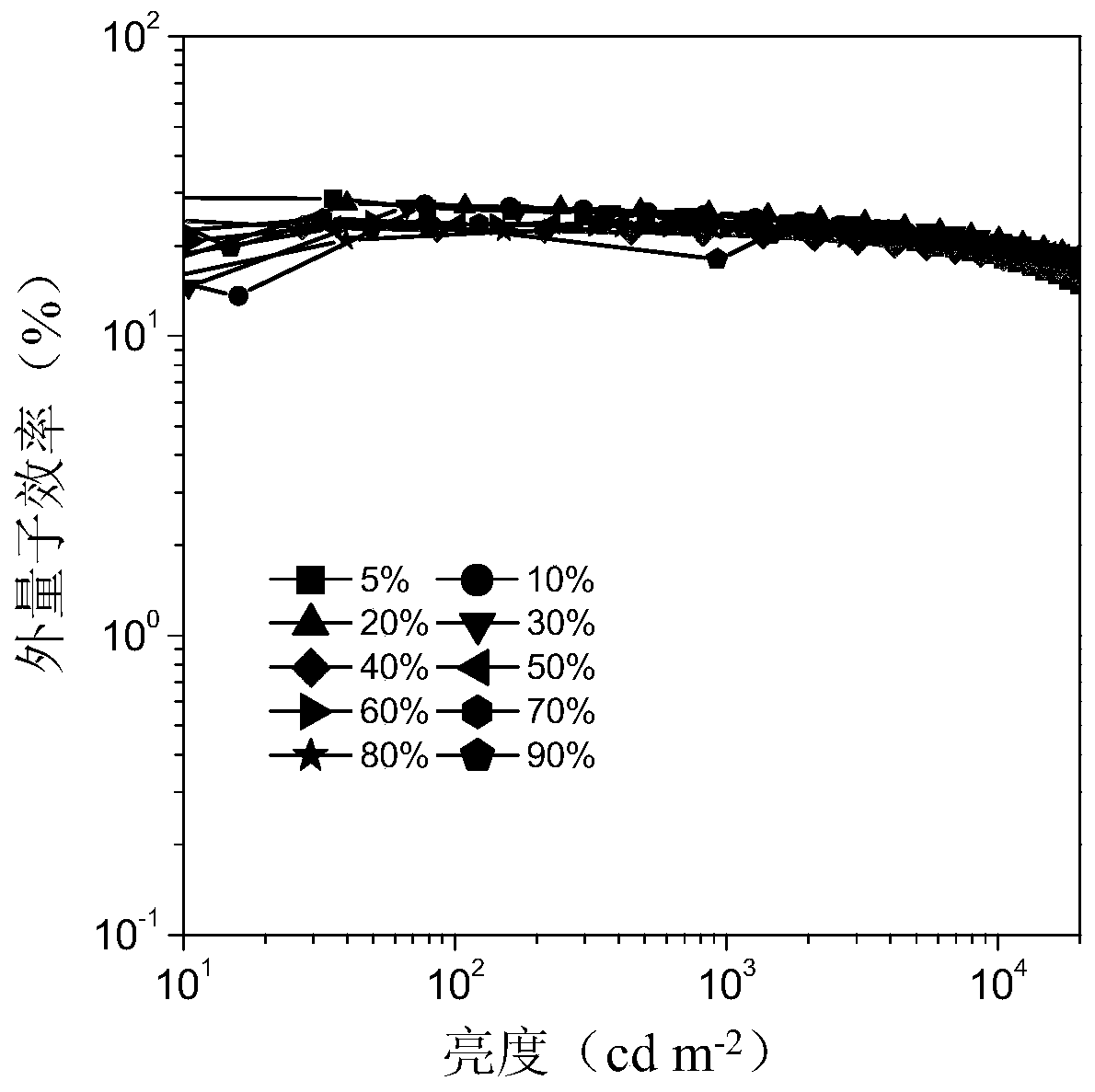
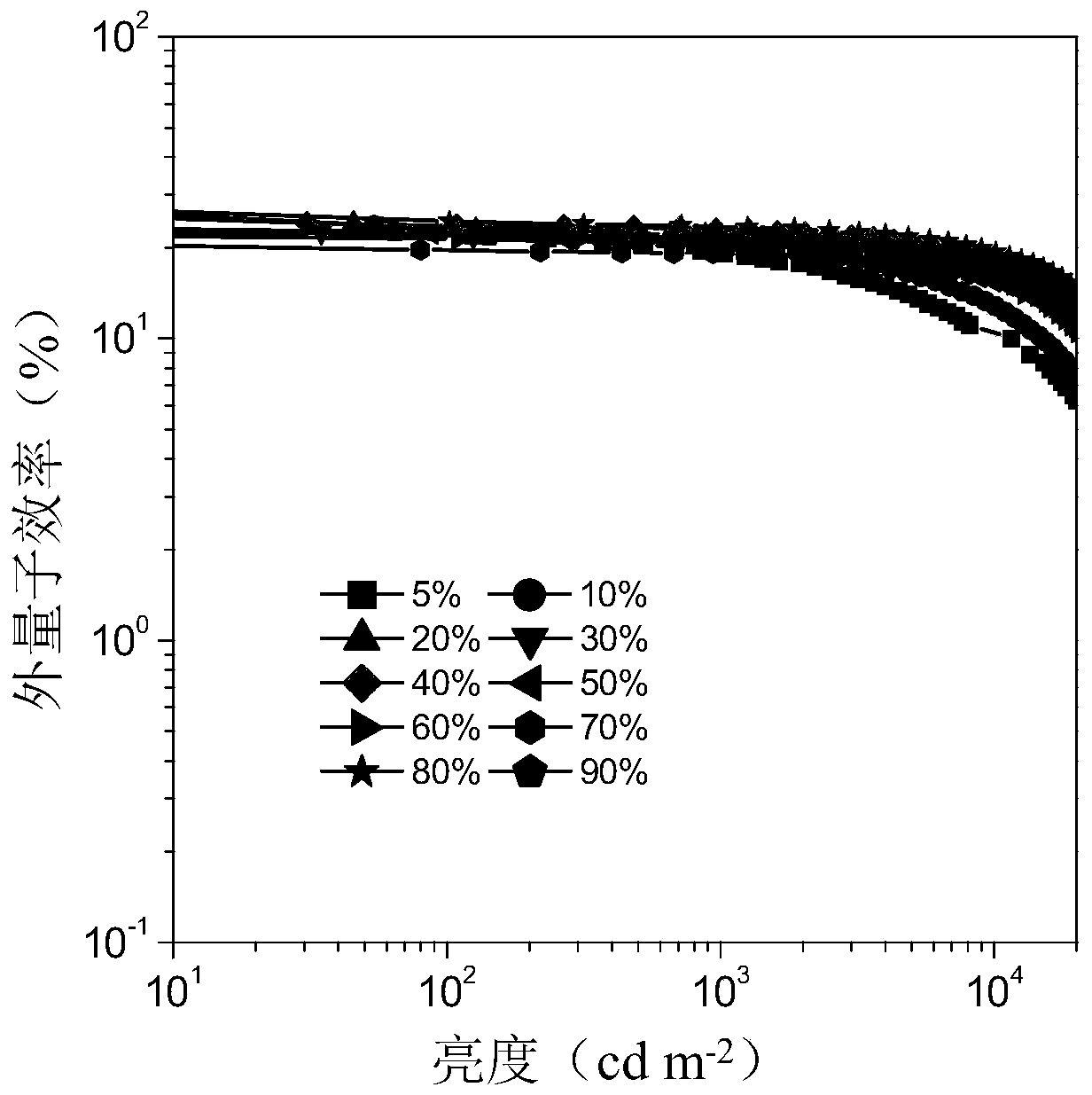
Examples
Embodiment 1
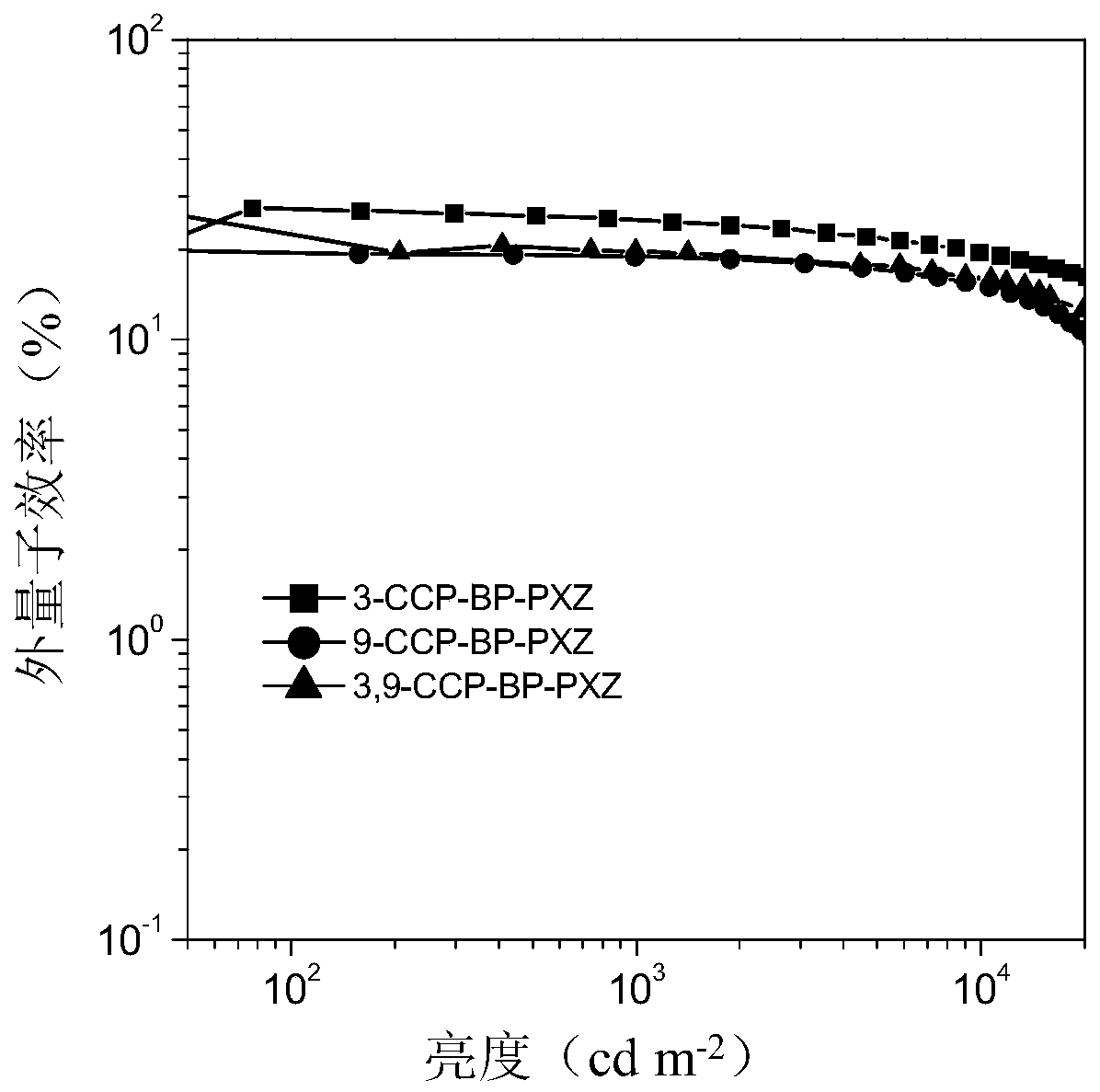
[0029] Example 1: Preparation of organic electroluminescent molecules (3-CCP-BP-PXZ) based on halogenated aromatic ketone derivatives
[0030]
[0031] synthetic route:
[0032]
[0033] (1) 3-chloro-N-phenylcarbazole (0.83g, 3.0mmol), p-fluorobenzoyl chloride (0.57g, 3.6mmol) and AlCl 3 (0.48g, 3.6mmol) was added to a 100mL two-necked flask, vacuumed, filled with N 2 (repeat 3 times), add ultra-dry dichloromethane (30 mL) under ice-bath conditions, heat up to 40° C., and react for 4 hours. Cooled to room temperature, quenched with dilute hydrochloric acid washing solution (30 mL), extracted with dichloromethane, dried over anhydrous magnesium sulfate, concentrated and passed through column to obtain white intermediate 2 with a yield of 88%.
[0034] (2) Add intermediate 2 (0.40g, 1.0mmol), phenoxazine (0.27g, 1.5mmol) and potassium tert-butoxide (0.22g, 2.0mmol) into a 100mL two-necked flask, pump and exchange gas 3 times, and protect with nitrogen DMF (20 mL) was ad...
Embodiment 2
[0036] Example 2: Preparation of organic electroluminescent molecules (9-CCP-BP-PXZ) based on halogenated aromatic ketone derivatives
[0037]
[0038] synthetic route:
[0039]
[0040] (1) 9-chloro-N-phenylcarbazole (0.83g, 3.0mmol), p-fluorobenzoyl chloride (0.57g, 3.6mmol) and AlCl 3 (0.48g, 3.6mmol) was added to a 100mL two-necked flask, vacuumed, filled with N 2 (repeat 3 times), add ultra-dry dichloromethane (30 mL) under ice-bath conditions, heat up to 40° C., and react for 4 hours. Cooled to room temperature, quenched with dilute hydrochloric acid washing solution (30 mL), extracted with dichloromethane, dried over anhydrous magnesium sulfate, concentrated and passed through column to obtain white intermediate 2 with a yield of 90%.
[0041] (2) Add intermediate 2 (0.40g, 1.0mmol), phenoxazine (0.27g, 1.5mmol) and potassium tert-butoxide (0.22g, 2.0mmol) into a 100mL two-necked flask, pump and exchange gas 3 times, and protect with nitrogen DMF (20 mL) was ad...
Embodiment 3
[0043] Example 3: Preparation of organic electroluminescent molecules (3,9-CCP-BP-PXZ) based on halogenated aromatic ketone derivatives
[0044]
[0045] synthetic route:
[0046]
[0047] (1) 3,9-dichloro-N-phenylcarbazole (0.93g, 3.0mmol), p-fluorobenzoyl chloride (0.57g, 3.6mmol) and AlCl 3 (0.48g, 3.6mmol) was added to a 100mL two-necked flask, vacuumed, filled with N 2 (repeat 3 times), add ultra-dry dichloromethane (30 mL) under ice-bath conditions, heat up to 40° C., and react for 4 hours. Cooled to room temperature, quenched with dilute hydrochloric acid washing solution (30 mL), extracted with dichloromethane, dried over anhydrous magnesium sulfate, concentrated and passed through column to obtain white intermediate 2 with a yield of 88%.
[0048] (2) Add intermediate 2 (0.43g, 1.0mmol), phenoxazine (0.27g, 1.5mmol) and potassium tert-butoxide (0.22g, 2.0mmol) into a 100mL two-necked flask, pump and exchange gas 3 times, and protect with nitrogen DMF (20 mL) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com