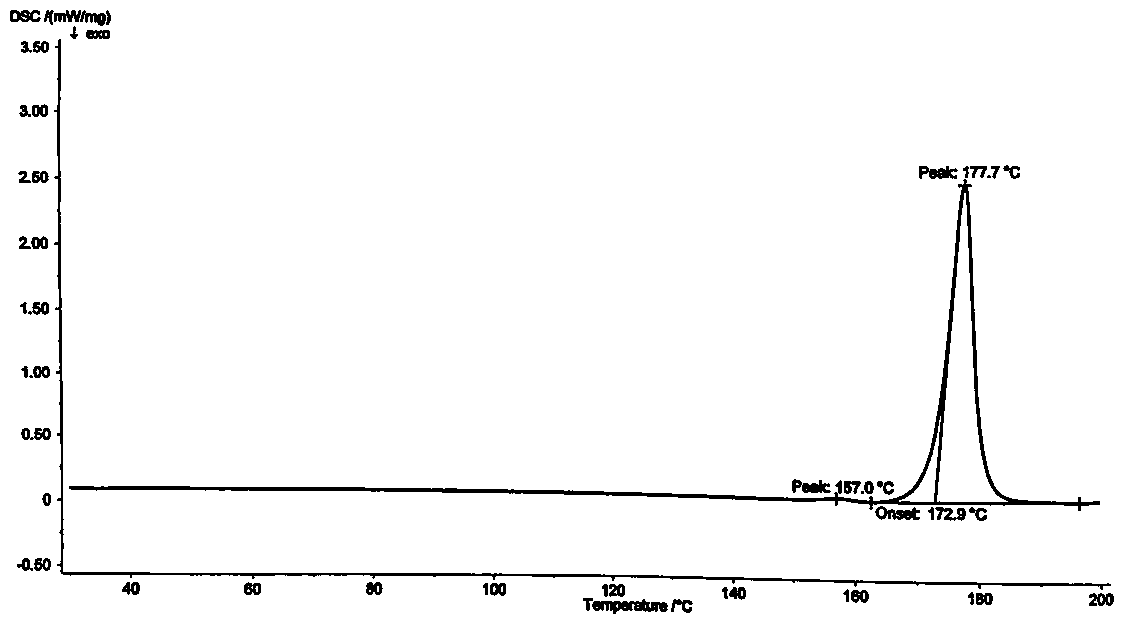
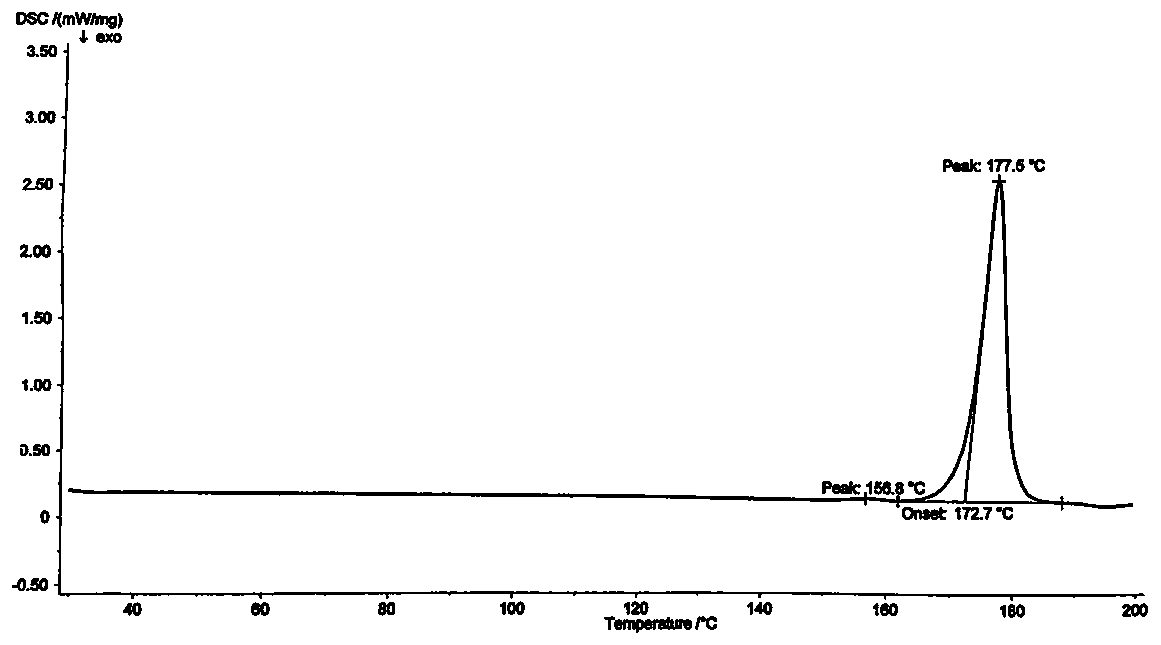
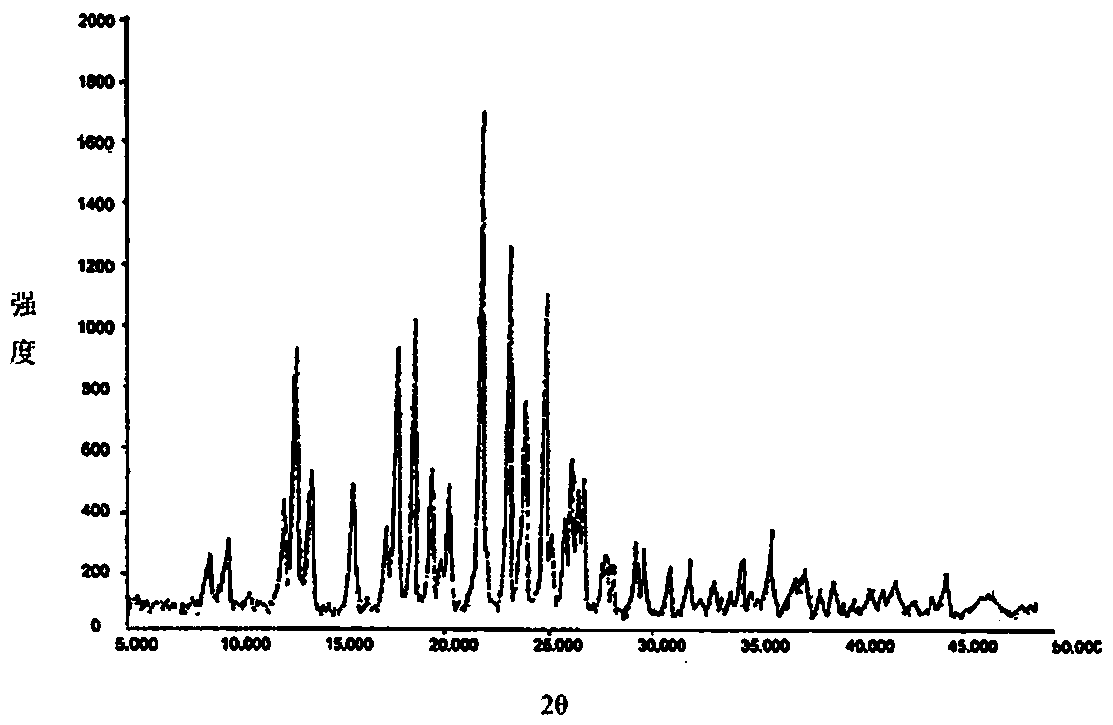
A kind of preparation method of clopidogrel hydrogen sulfate crystal form ii
A technology for clopidogrel hydrogen sulfate and clopidogrel free base is applied in the directions of organic chemistry, organic chemistry and the like, and can solve the problems of complex clopidogrel hydrogen sulfate process, harsh reaction conditions and high impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The present invention provides a kind of preparation method of clopidogrel bisulfate crystal form II, which comprises the following steps:
[0041] S1. Preparation of (+) o-chlorophenylglycine methyl ester
[0042] Add extractant, water, and inorganic base into the reaction vessel, stir to dissolve, then add (+) o-chlorophenylglycine methyl ester tartrate, separate the liquid after the reaction is complete, and extract the water layer with the extractant. The organic layers were combined, dried with a desiccant, centrifuged, and the filtrate was taken to reclaim the extractant under reduced pressure to obtain (+) o-chlorophenylglycine methyl ester. The reaction process is as follows:
[0043]
[0044] In certain embodiments of the present invention, the inorganic base is selected from dipotassium hydrogen phosphate, sodium sulfate, sodium carbonate, potassium carbonate, or disodium hydrogen phosphate. The inorganic base is used as an acid-binding agent in the prepar...
Embodiment 1
[0076] Add 300ml of dichloromethane, 160ml of water, and 80g of sodium carbonate into the three-necked flask, stir to dissolve, then add 140g of (+) o-chlorophenylglycine methyl ester tartrate, separate the liquids after the reaction is complete, and extract the water layer with 50ml of dichloromethane Three times, the organic layers were combined, dried with anhydrous sodium sulfate, centrifuged, and the filtrate was taken to recover dichloromethane under reduced pressure to obtain (+) o-chlorophenylglycine methyl ester.
[0077] Add 600ml of acetonitrile and 140g of dipotassium hydrogen phosphate to the three-necked flask, mix with (+) o-chlorophenylglycine methyl ester obtained in the previous step, and stir at 20°C for 1 hour. Then add 170 g of 2-(2-thienyl)ethanol p-toluenesulfonate, then raise the temperature to 48° C., and keep the temperature for reaction. Centrifuge after the reaction, collect the mother liquor into the reaction vessel, add appropriate amount of hydro...
Embodiment 2
[0098] Add 300ml of dichloromethane, 160ml of water, and 80g of sodium carbonate into the three-necked flask, stir to dissolve, then add 140g of (+) o-chlorophenylglycine methyl ester tartrate, separate the liquids after the reaction is complete, and extract the water layer with 50ml of dichloromethane Three times, the organic layers were combined, dried with anhydrous sodium sulfate, centrifuged, and the filtrate was taken to recover dichloromethane under reduced pressure to obtain (+) o-chlorophenylglycine methyl ester.
[0099] Add 600ml of acetonitrile and 140g of dipotassium hydrogen phosphate to the three-necked flask, mix with (+) o-chlorophenylglycine methyl ester obtained in the previous step, and stir at 30°C for 1 hour. Then add 170 g of 2-(2-thienyl)ethanol p-toluenesulfonate, then raise the temperature to 52° C., and keep the temperature for reaction. Centrifuge after the reaction, collect the mother liquor into the reaction vessel, add appropriate amount of hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com