Pazufloxacin mesilate for injection and preparation method
A technology of pazufloxacin mesylate and injection, which is applied in the field of pharmaceutical preparations, can solve problems such as hypoglycemia, electrolyte disturbance, and poor water solubility, and achieve the effects of reducing hypoglycemia, reducing electrolyte disturbance, and avoiding precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of pazufloxacin mesylate for injection comprises the following steps:
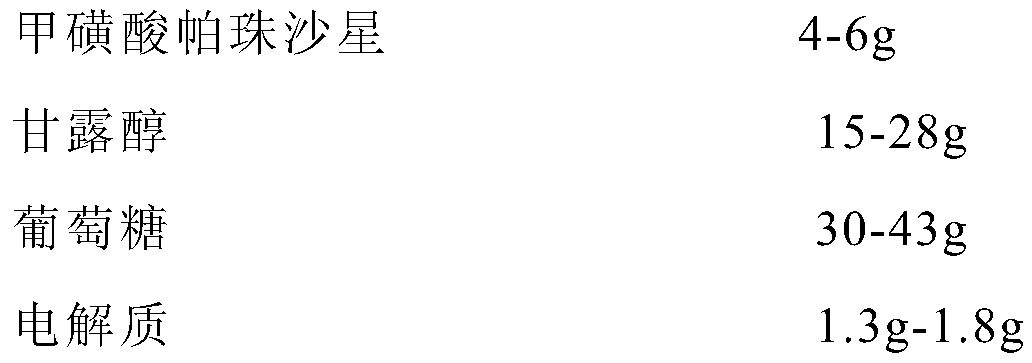
[0023] S1: Weigh 350mL of water for injection, cool to below 70°C, add 30g of glucose and 15g of mannitol, and stir to dissolve;
[0024] S2: Weigh 350mL water for injection, cool to below 55°C, add 0.6g sodium chloride, 0.5g potassium chloride, 0.2g magnesium chloride, and stir evenly;
[0025] S3: Combine the injections in steps S1 and S2, add 4g of pazufloxacin mesylate, stir well, add methanesulfonic acid, adjust the pH to 3.3-4.1, add water for injection to 1000mL, filter with activated carbon for injection Post-potting encapsulation;
[0026] S4: The filtered injection is freeze-dried in a freeze-drying machine to obtain a freeze-dried powder, which is subpackaged, boxed, and packaged.
Embodiment 2
[0027] Embodiment 2: the preparation of pazufloxacin mesylate for injection comprises the following steps:
[0028] S1: Weigh 350mL of water for injection, cool to below 70°C, add 43g of glucose and 28g of mannitol, stir to dissolve;
[0029] S2: Weigh 350mL water for injection, cool to below 55°C, add 0.8g sodium chloride, 0.7g potassium chloride, 0.3g magnesium chloride, and stir evenly;
[0030] S3: Combine the injections in steps S1 and S2, add 6g of pazufloxacin mesylate, stir well, add methanesulfonic acid, adjust the pH to 3.3-4.1, add water for injection to 1000mL, filter with activated carbon for injection ;
[0031] S4: The filtered injection is freeze-dried in a freeze-drying machine to obtain a freeze-dried powder, which is subpackaged, boxed, and packaged.
Embodiment 3
[0032] Embodiment 3: the preparation of pazufloxacin mesylate for injection comprises the following steps:
[0033] S1: Weigh 350mL of water for injection, cool to below 70°C, add 37g of glucose and 22g of mannitol, stir to dissolve;
[0034] S2: Weigh 350mL water for injection, cool to below 55°C, add 0.7g sodium chloride, 0.6g potassium chloride, 0.2g magnesium chloride, and stir evenly;
[0035] S3: Combine the injections in steps S1 and S2, add 5g of pazufloxacin mesylate, stir well, add methanesulfonic acid, adjust the pH to 3.3-4.1, add water for injection to 1000mL, filter with activated carbon for injection ;
[0036] S4: The filtered injection is freeze-dried in a freeze-drying machine to obtain a freeze-dried powder, which is subpackaged, boxed, and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com