Te-doped A2SnCl6 perovskite material and preparation method thereof
A technology of perovskite material and charging ratio, which is applied in luminescent materials, chemical instruments and methods, inorganic chemistry, etc., can solve problems such as failure to meet the requirements of device application, yellow-green light-emitting materials have not yet been reported, etc., and achieves low production cost, Beneficial for mass production, high quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Configuration of Cs precursor: Dissolve 10mmol CsCl in 10mL concentrated HCl solvent, ultrasonic
[0041] Dissolved to obtain a 1mol / L colorless, clear and transparent Cs precursor solution;
[0042] (2) Configuration of Sn precursor: 5mmol SnCl 4 Dissolved in 9.415 mL of concentrated HCl solvent,
[0043] Ultrasonic dissolution to obtain a 0.5mol / L colorless, clear and transparent Sn precursor solution;
[0044] (3) Configuration of Te precursor: 0.1mmol Na 2 TeO 3 Dissolve in 10mL concentrated HCl solvent,
[0045] Ultrasonic dissolution, to obtain a yellow clear solution;
[0046] (4) Dissolution: Measure 1mL of the Sn precursor solution prepared in step (2) and add 20mL of high-temperature reaction
[0047] Then add 2.5mL Te precursor solution prepared in step (3), and then add 0.5mL concentrated HCl; finally add 1mL Cs precursor prepared in step (1), immediately A suspension is formed;
[0048] (5) Heating: Seal the reactor tightly and put it in an oven...
Embodiment 2
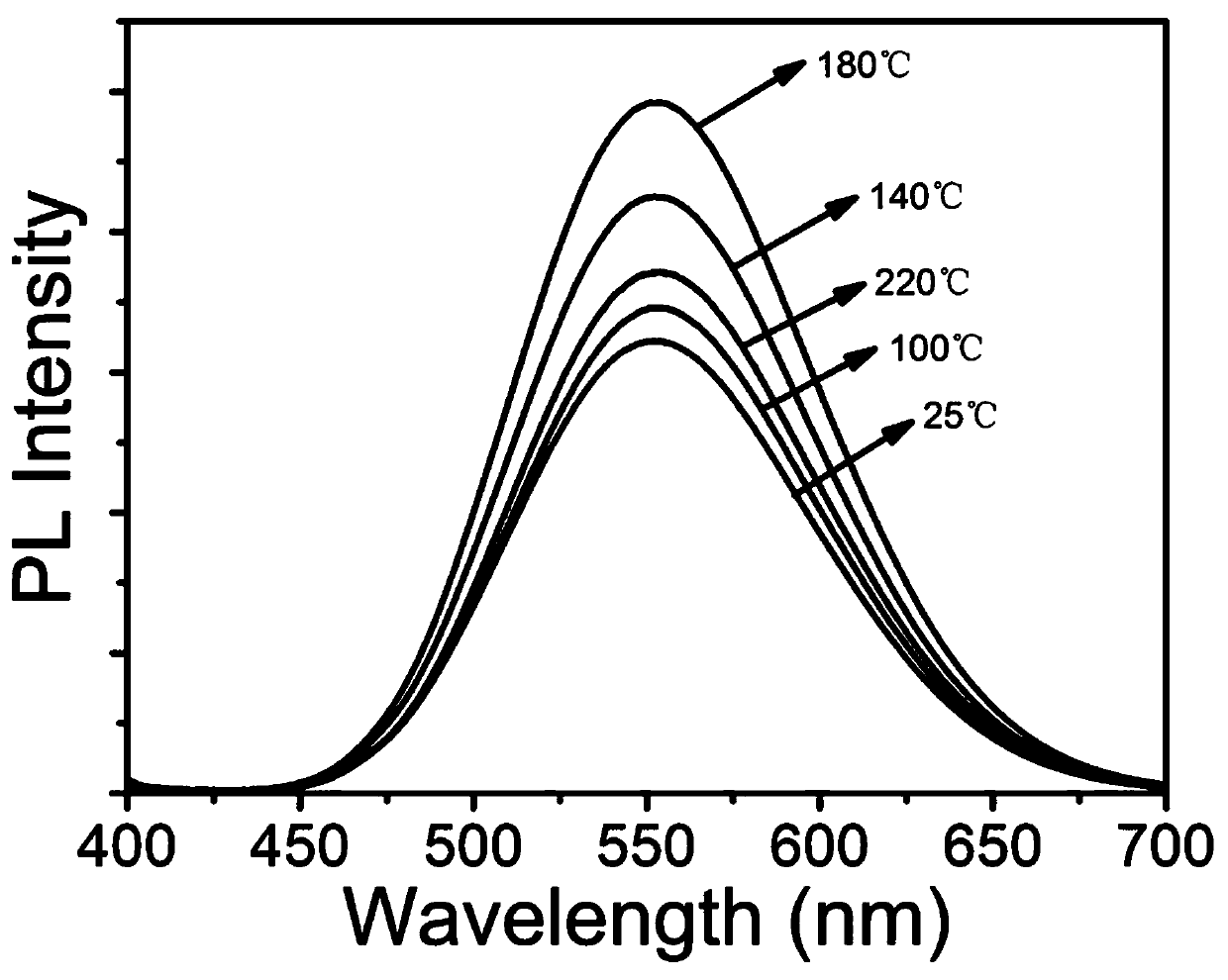
[0055] The difference from Example 1 is that the reaction temperature is 25°C, 100°C, 140°C, 180°C, 220°C, and the others are the same as in Example 1.
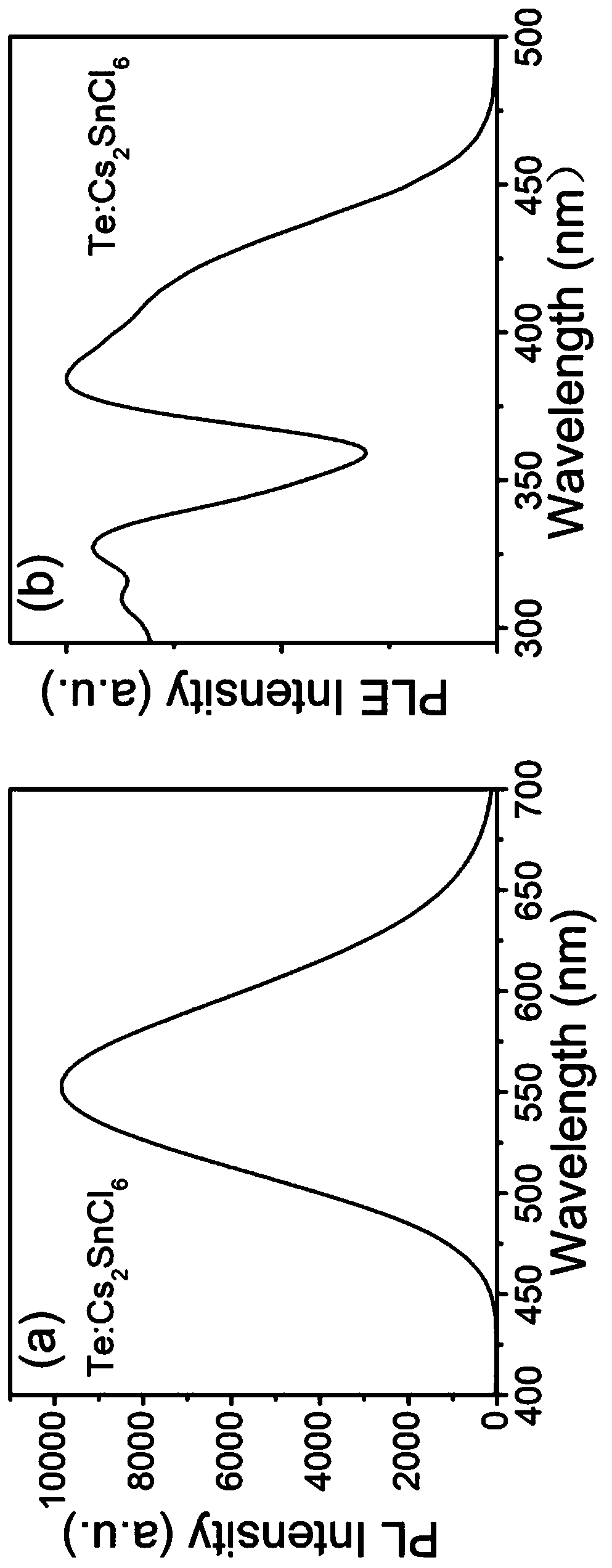
[0056] The Te-doped Cs that embodiment 2 prepares 2 SnCl 6 The emission (PL) spectrum of the perovskite material is shown in image 3 As shown, the results show that even at the reaction temperature of room temperature (25°C), the fluorescence quantum yield is as high as 53.3%. The best value is 81.5%.
Embodiment 3
[0058] The configuration of Rb precursor: Dissolve 10mmol RbCl in 10mL concentrated HCl solvent, and ultrasonically dissolve to obtain a 1mol / L colorless, clear and transparent Rb precursor solution;
[0059] The only difference from Example 1 is that in this example, 1 mL of Rb precursor solution is added to the mixed solution instead of the Cs precursor, and the others are the same as in Example 1.
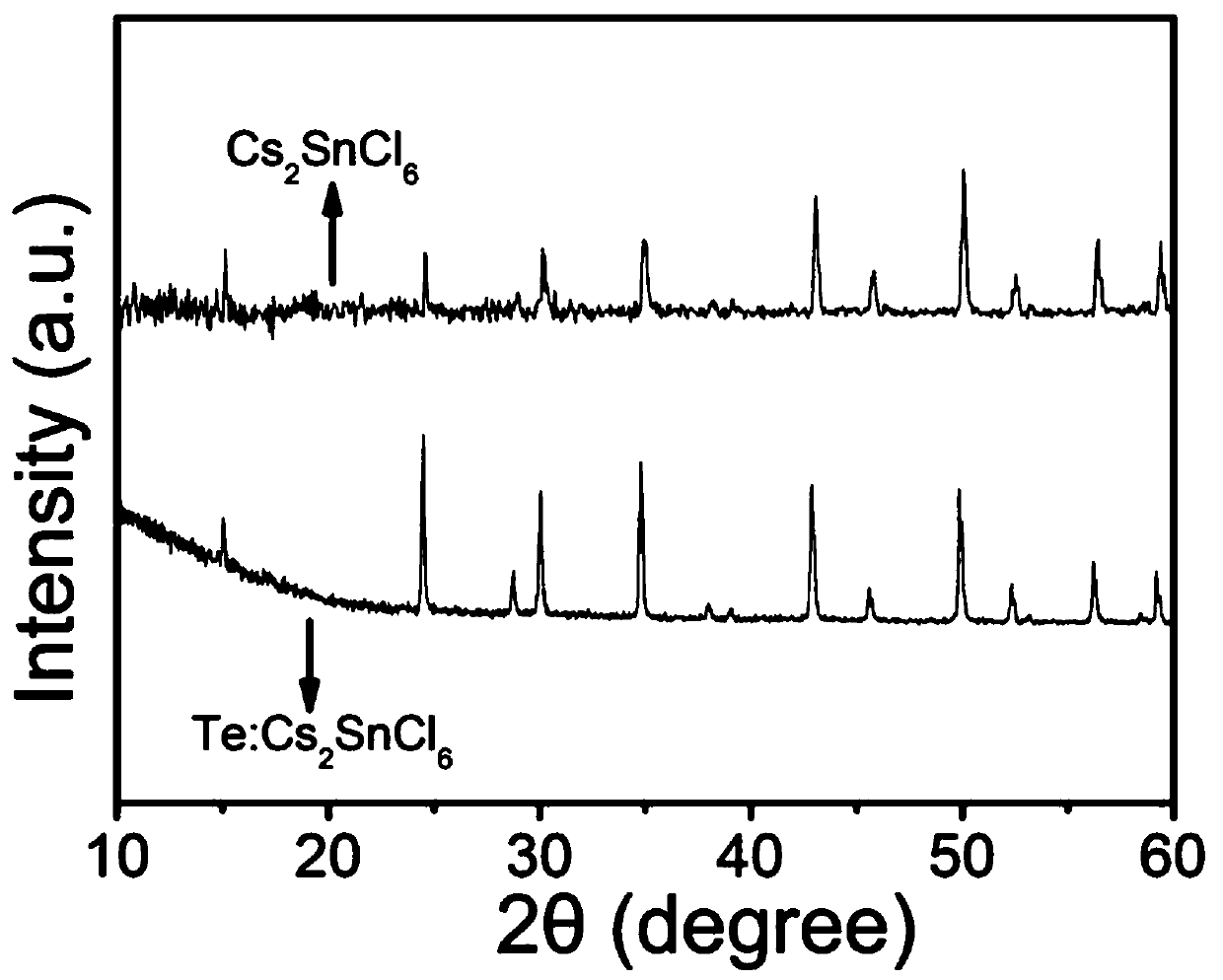
[0060] The Te-doped Rb that embodiment 3 prepares 2 SnCl 6 The emission (PL) spectrum of the perovskite material is shown in Figure 4 As shown in a, the results show that Te doped Rb 2 SnCl 6 The emission peak of perovskite is at 548nm, Figure 4 b is the corresponding XRD pattern, indicating that the as-prepared Te-doped Rb 2 SnCl 6 Perovskite materials have a cubic phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com