Photo-catalytic preparation method of bibenzyl compounds
A technology of bibenzyl compounds and compounds, which is applied in the field of artificial synthesis of bibenzyl compounds, can solve the problems that cannot be widely used in the synthesis of bibenzyl compounds, and achieve the effect of low cost and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
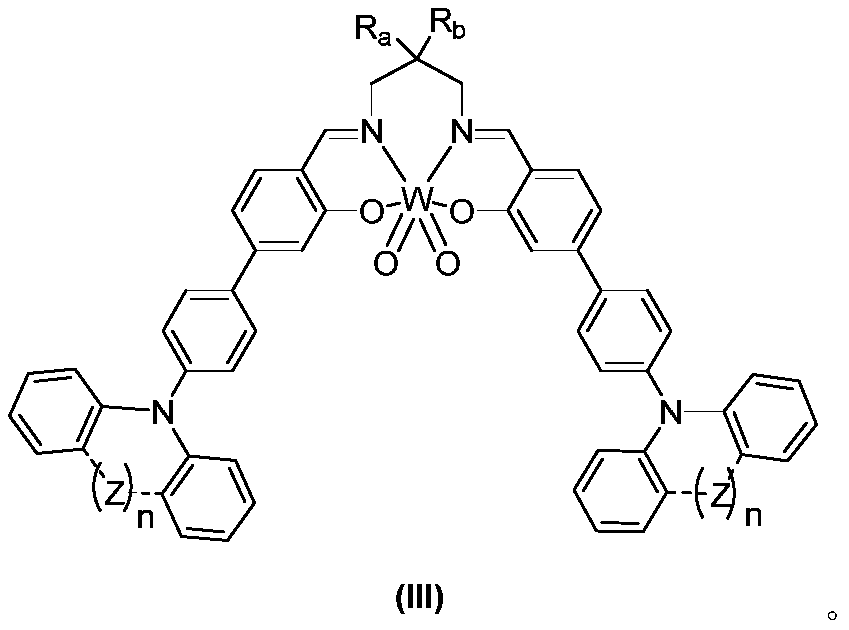
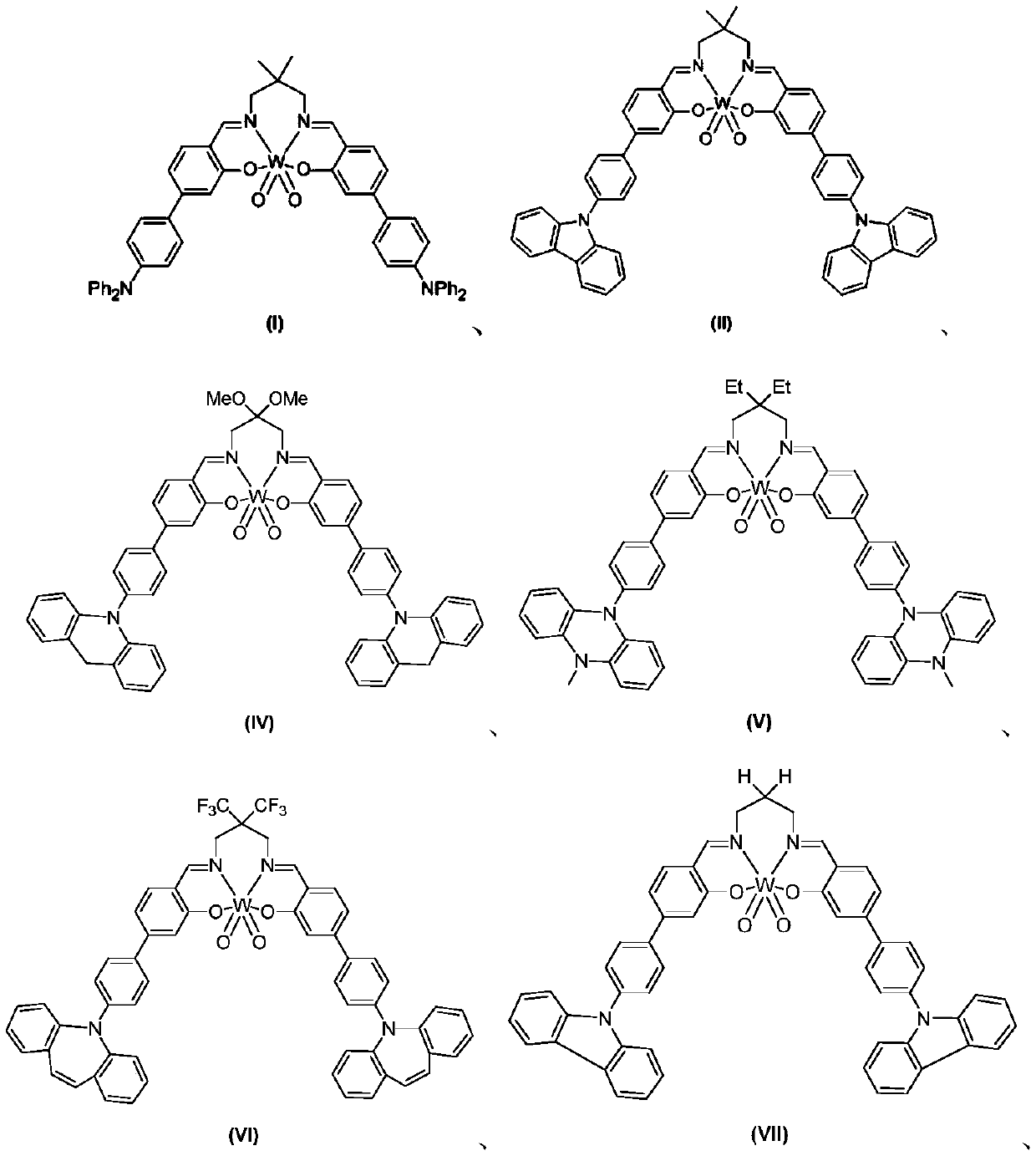
[0039] In a 10mL 15x 25mm test tube with a stir bar, add organic tungsten photocatalyst (I) (1.0 mol%), alkali, additives, solvent, and then add benzyl bromide (0.5 mmol), stopper with rubber stopper, and argon drum The bubble removes oxygen for 15 minutes. After irradiating with 410nm LEDs for 12 hours, the color of the solution became colorless. Then add 10 mL of water, extract twice with dichloromethane (20 mL), dry with anhydrous sodium sulfate, separate by column chromatography, and rinse with ethyl acetate / petroleum ether (EA / PE=1:10) to obtain 1,2 -Diphenylethane, its nuclear magnetic analysis data is: 1 H NMR(400MHz, CDCl 3 )δ7.56–7.28(m,4H), 7.23(d,J=7.3Hz,6H), 2.97(s,4H); 13 CNMR(101MHz, CDCl 3 )δ141.82,128.48,128.37,125.95,37.99ppm.
[0040] Table 1: Conditional screening optimization
[0041]
[0042] a Use dibromomethane coordinate sample calculation 1 Product yield in H NMR
Embodiment 2
[0044] In a 10mL 15x 25mm test tube with a stir bar, add organic tungsten photocatalyst (I), TBAB (0.5mmol, 1.0eq), B 2 Pin 2 (0.55mmol), K 2 CO 3 (0.5mmol, 1eq), add 5mL acetonitrile, then add benzyl bromide (0.5mmol), put a rubber stopper, and bubbling argon to remove oxygen for 15min. Irradiated with 410nm LEDs for 12 hours, the color of the solution became colorless. Then add 10 mL of water, extract twice with dichloromethane (20 mL), dry with anhydrous sodium sulfate, separate by column chromatography, and rinse with ethyl acetate / petroleum ether (EA / PE=1:10) to obtain 1,2 -Diphenylethane.
[0045] Different equivalents of organic tungsten photosensitizers were used to catalyze benzyl bromide. The results are shown in Table 1:
[0046] Table 2: Yield under different catalyst usage
[0047] Serial number The amount of catalyst Product yield 1 0.2mol%81% 2 0.3mol%84% 3 0.5mol%92% 4 0.8mol%93% 5 1.0mol%95% 6 1.2mol%97% 7 1.5mol%98% 8 1.6mol%98% 9 1.8mol%99%
[0048] It can be...
Embodiment 3
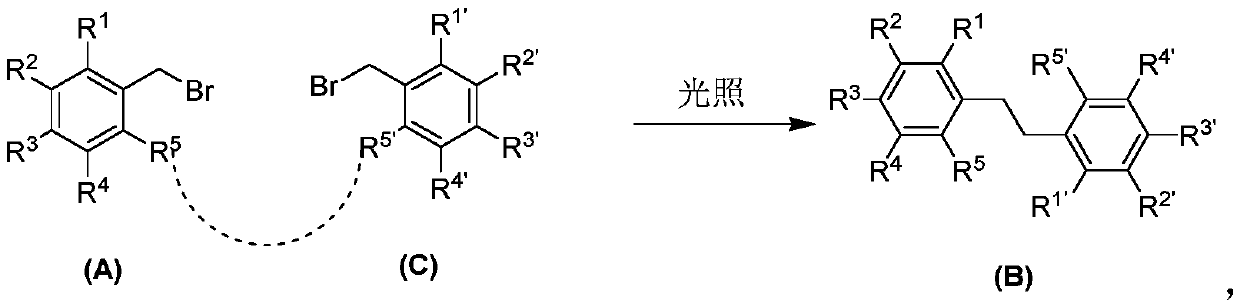
[0050] In a 10mL 15x 25mm test tube with a stir bar, add organic tungsten photocatalyst (I) (1mol%), TBAB (0.5mmol, 1.0eq), B 2 Pin 2 (0.55mmol), K 2 CO 3 (0.5mmol, 1eq), add 5mL acetonitrile, then add the benzyl bromide compound (0.5mmol) represented by formula (A), put a rubber stopper, and bubbling argon to remove oxygen for 15min. Irradiated with 410nm LEDs for 12 hours, the color of the solution became colorless. Then add 10 mL of water, extract twice with dichloromethane (20 mL), dry with anhydrous sodium sulfate, separate by column chromatography, and rinse with ethyl acetate / petroleum ether (EA / PE=1:10) to obtain the corresponding product .
[0051] Table 3: Synthesis of (B2), (B4), (B5), (B6) and (B9) under the action of organotungsten photocatalyst (I)
[0052]
[0053] (B2): 1 H NMR(500MHz, CDCl 3 )δ7.53(d,J=8.1Hz,4H), 7.20(d,J=8.1Hz,4H), 2.97(s,4H); 13 C NMR (126MHz, CDCl3) δ 146.17, 132.27, 129.28, 118.84, 110.11, 37.16ppm.
[0054] (B4): 1 H NMR(500MHz, CDCl 3 )δ7.56(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com