Two-stage targeted polymeric pro-drug for treating acute kidney injury and preparation method
A technology for acute kidney injury and prodrugs, applied in the field of new drug delivery system research, can solve the problems of poor water solubility, poor stability, and limited clinical application, and achieve the effect of reversing oxidative stress damage and increasing distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
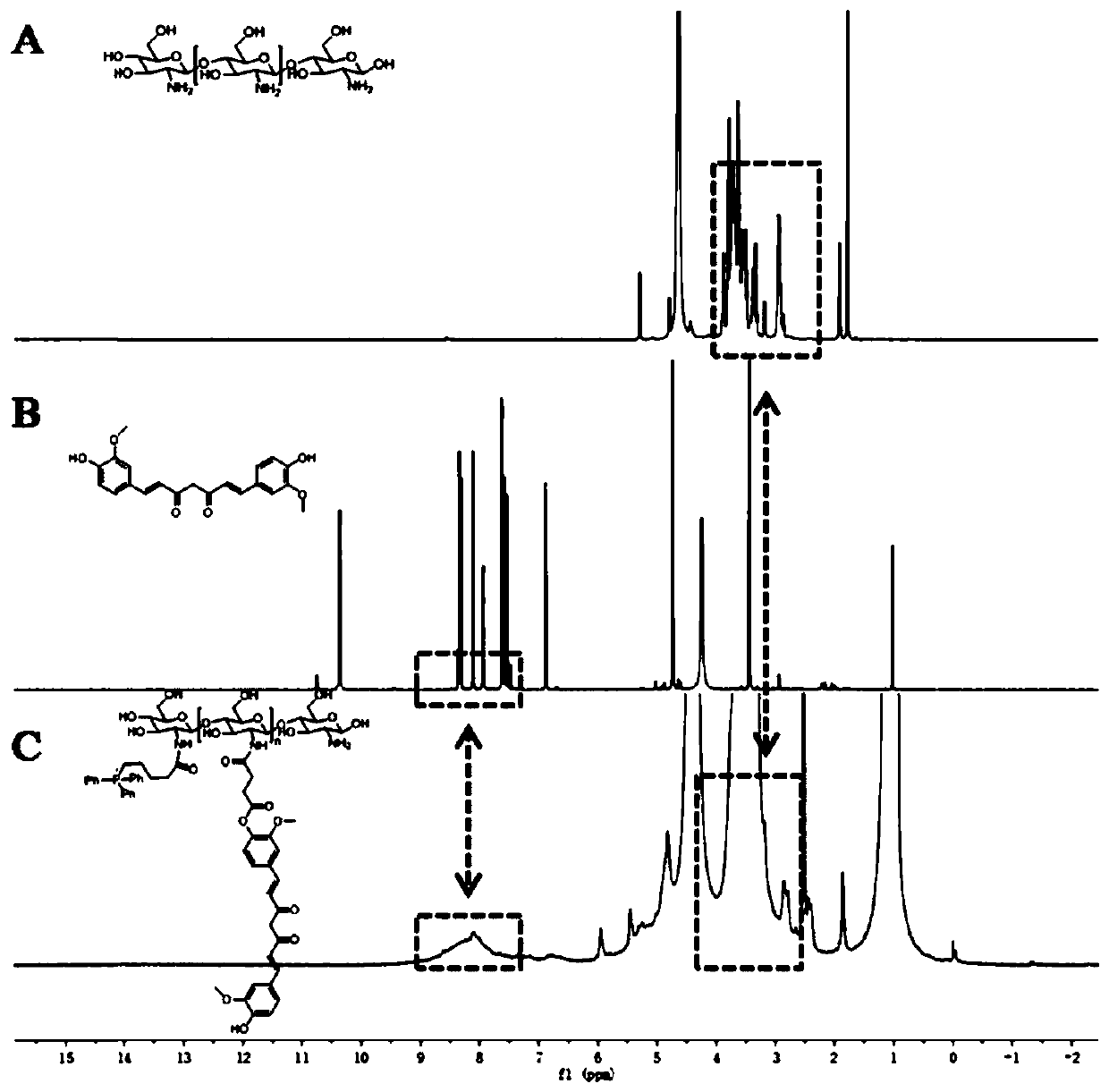
Image
Examples
Embodiment 1
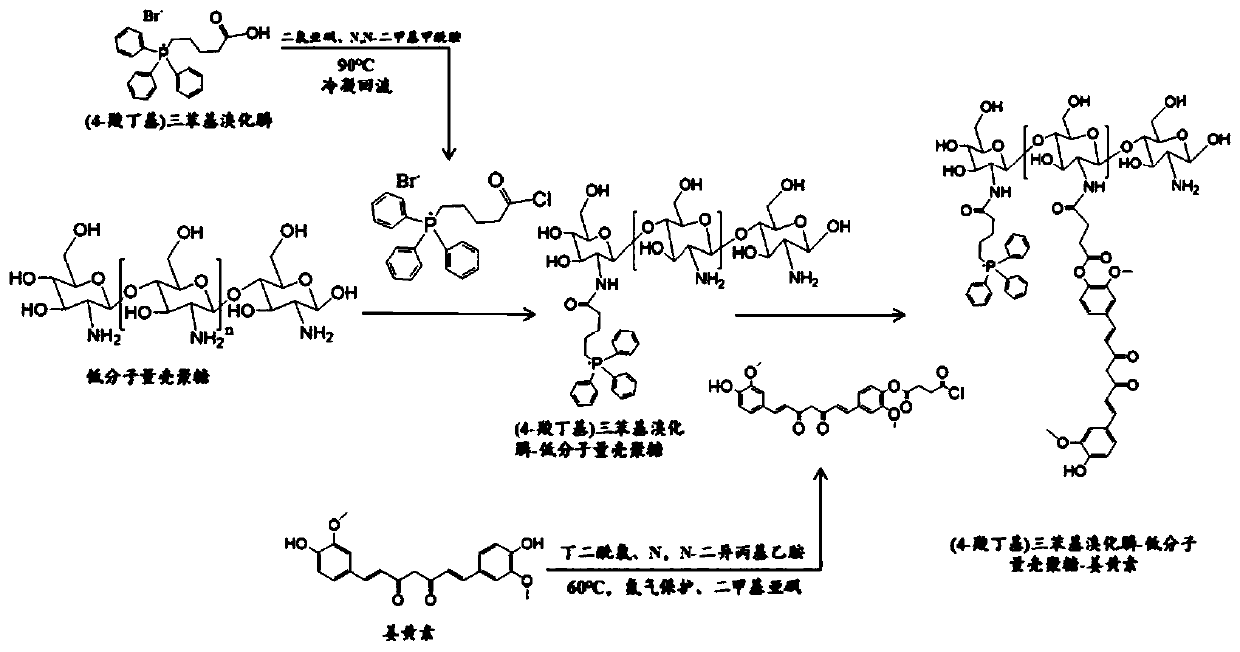
[0040] 1. Synthesis of (4-carboxybutyl)triphenylphosphonium bromide-low molecular weight chitosan graft
[0041] Weigh 0.8866g (4-carboxybutyl) triphenylphosphonium bromide and add it to 10mL thionyl chloride, then add 50 μL N,N-dimethylformamide, stir at 90℃ to completely dissolve and condense the reactants Reflux and react for 4h to activate the carboxyl group of (4-carboxybutyl)triphenylphosphonium bromide. After the solvent is removed by rotary evaporation, 6.448g of low molecular weight chitosan (polymerization degree 40) is added to the round bottom flask and added 10mL anhydrous dimethyl sulfoxide solution, continue to react for 48h. After the reaction, the reaction product was placed in a dialysis bag with a molecular weight cut-off of 1000, and the dialysis was continued with pure water for 48 hours. The suspension in the dialysis bag was collected, centrifuged at 14000 rpm for 10 min, and the supernatant was taken to freeze-dry to obtain (4-carboxybutyl)triphenylphosph...
Embodiment 2
[0044] 1. Synthesis of (4-carboxybutyl)triphenylphosphonium bromide-low molecular weight chitosan graft
[0045] Weigh 0.8866g (4-carboxybutyl) triphenylphosphonium bromide and add it to 10mL thionyl chloride, then add 50 μL N,N-dimethylformamide, stir at 90℃ to completely dissolve and condense the reactants Reflux and react for 4h to activate the carboxyl group of (4-carboxybutyl)triphenylphosphonium bromide. After the solvent is removed by rotary evaporation, 4.836g of low molecular weight chitosan (polymerization degree 30) is added to the round bottom flask and added 10mL anhydrous dimethyl sulfoxide solution, continue to react for 48h. After the reaction, the reaction product was placed in a dialysis bag with a molecular weight cut-off of 1000, and the dialysis was continued with pure water for 48 hours. The suspension in the dialysis bag was collected, centrifuged at 14000 rpm for 10 min, and the supernatant was taken to freeze-dry to obtain (4-carboxybutyl)triphenylphosph...
Embodiment 3
[0048] 1. Synthesis of (4-carboxybutyl)triphenylphosphonium bromide-low molecular weight chitosan graft
[0049] Weigh 0.8866g (4-carboxybutyl) triphenylphosphonium bromide and add it to 10mL thionyl chloride, then add 50 μL N,N-dimethylformamide, stir at 90℃ to completely dissolve and condense the reactants Reflux and react for 4h to activate the carboxyl group of (4-carboxybutyl)triphenylphosphonium bromide. After the solvent is removed by rotary evaporation, 3.224g of low molecular weight chitosan (polymerization degree 20) is added to the round bottom flask and added 10mL anhydrous dimethyl sulfoxide solution, continue to react for 48h. After the reaction, the reaction product was placed in a dialysis bag with a molecular weight cut-off of 1000, and the dialysis was continued with pure water for 48 hours. The suspension in the dialysis bag was collected, centrifuged at 14000 rpm for 10 min, and the supernatant was taken to freeze-dry to obtain (4-carboxybutyl)triphenylphosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com