Method for inactivating viruses in plasma
A technology of virus inactivation and blood plasma, applied in disinfection, irradiation, chemistry, etc., to achieve the effect of overcoming thermal effects and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 main reagent, raw material and instrument
[0033] The plasma used in the experiment was fresh frozen dog plasma purchased from Beijing Tianqin Yihe Biotechnology Co., Ltd.;
[0034] Pseudorabies virus and encephalomyocarditis virus were purchased from China Veterinary Microbiological Culture Collection and Management Center;
[0035] PK-15 and BHK-21 cells were purchased from China Veterinary Microbiology Culture Collection and Management Center;
[0036] Medicinal rutin is produced by Sichuan Xieli Pharmaceutical Co., Ltd.;
[0037] Rutin is produced by SIGMA / MERCK;
[0038] The plasma virus inactivator was developed by Zhongke Shisheng (Beijing) Pharmaceutical Technology Co., Ltd.;
[0039] The rest of the reagents and instruments are conventional domestic models.
Embodiment 2
[0040] The virus inactivation and detection of embodiment 2 blood plasma
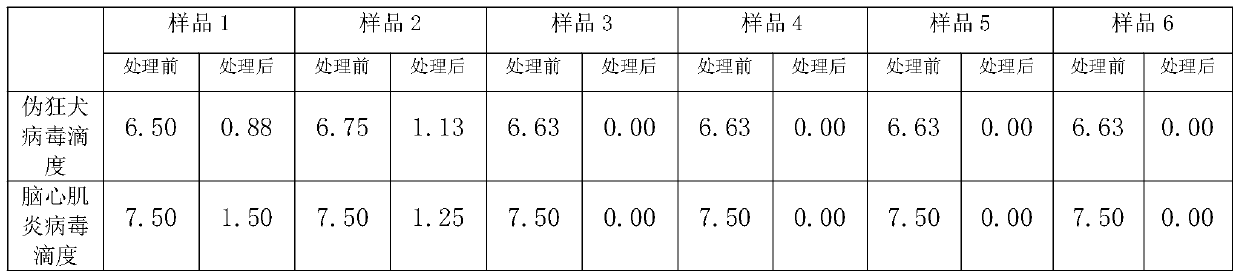
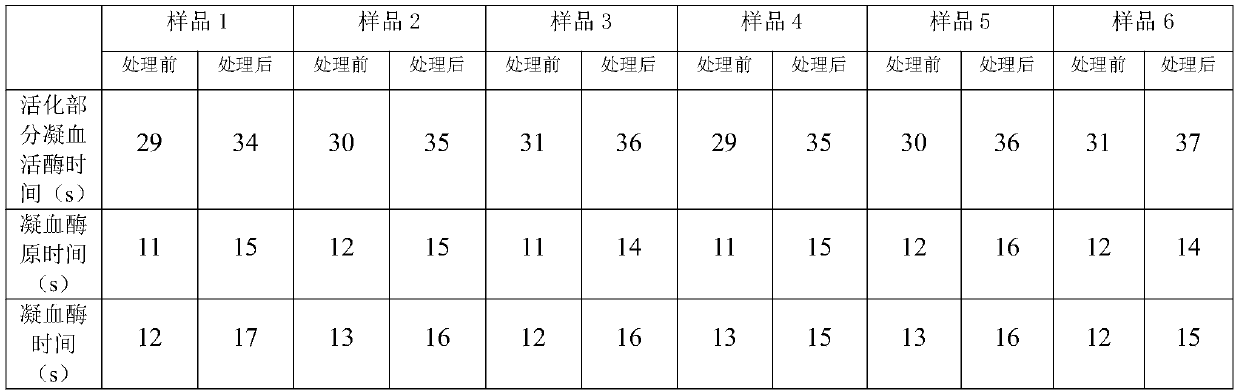
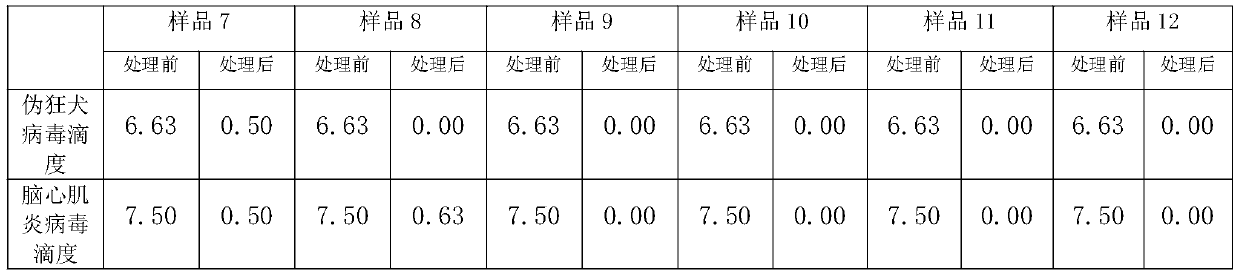
[0041] 1 Preparation of poisoned plasma: Thaw frozen plasma, take 100ML and put it in a plasma bag, add medicinal rutin or rutin in proportion, and then mix the indicator virus liquid and plasma at a ratio of 1:9; with capsule indication Pseudorabies virus (PRV) was selected as the virus, and encephalomyocarditis virus (EMCV) was selected as the non-enveloped indicator virus.
[0042] 2 inactivation process: use 25 # The silicone tube connects the plasma bag with the sample inlet of the plasma virus inactivation instrument, and the sample outlet is also connected with 25 # Connect the silicone tube to the plasma collection bag; turn on the UVC light source (UVC intensity: 500-5000μw / cm 2 ) After the light intensity is stable, turn on the power of the peristaltic pump, pump the poisoned plasma into the spiral quartz glass inactivation tube, so that the plasma is completely treated with radiation inacti...
Embodiment 3
[0052] Each group of sample processing of embodiment 3
[0053] Due to space reasons, all combinations of proportional concentration orthogonal experiments are not shown, and only some representative results are shown to show the basic trend. Plasma was exposed to the virus in proportion, each sample was added with different amounts of medicinal rutin or rutin, and different irradiation methods were used to verify the inactivation effect on pseudorabies virus and encephalomyocarditis virus.
[0054] 1 The inactivation treatment method of adding medicinal rutin as a protective agent and enhancer is as follows:
[0055] (Sample 1) Add rutin injection 0.3g / L to the poisoned plasma, mix well and put it into the inactivation instrument. 2 , irradiation treatment for 1200s.
[0056] (Sample 2) Add rutin injection 0.3g / L to the poisoned plasma, put it into the inactivator after mixing, and put it into the inactivation instrument under the light intensity of 5000μw / cm 2 , irradiati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com