A-pi-D-pi-A type organic small molecule based on diindenopyrazine as well as preparation method and application thereof
A technology of indenopyrazine and small molecules, applied in the field of A-π-D-π-A type organic small molecules and their preparation, to achieve the effects of wide supply of raw materials, high promotion, and high short-circuit current
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
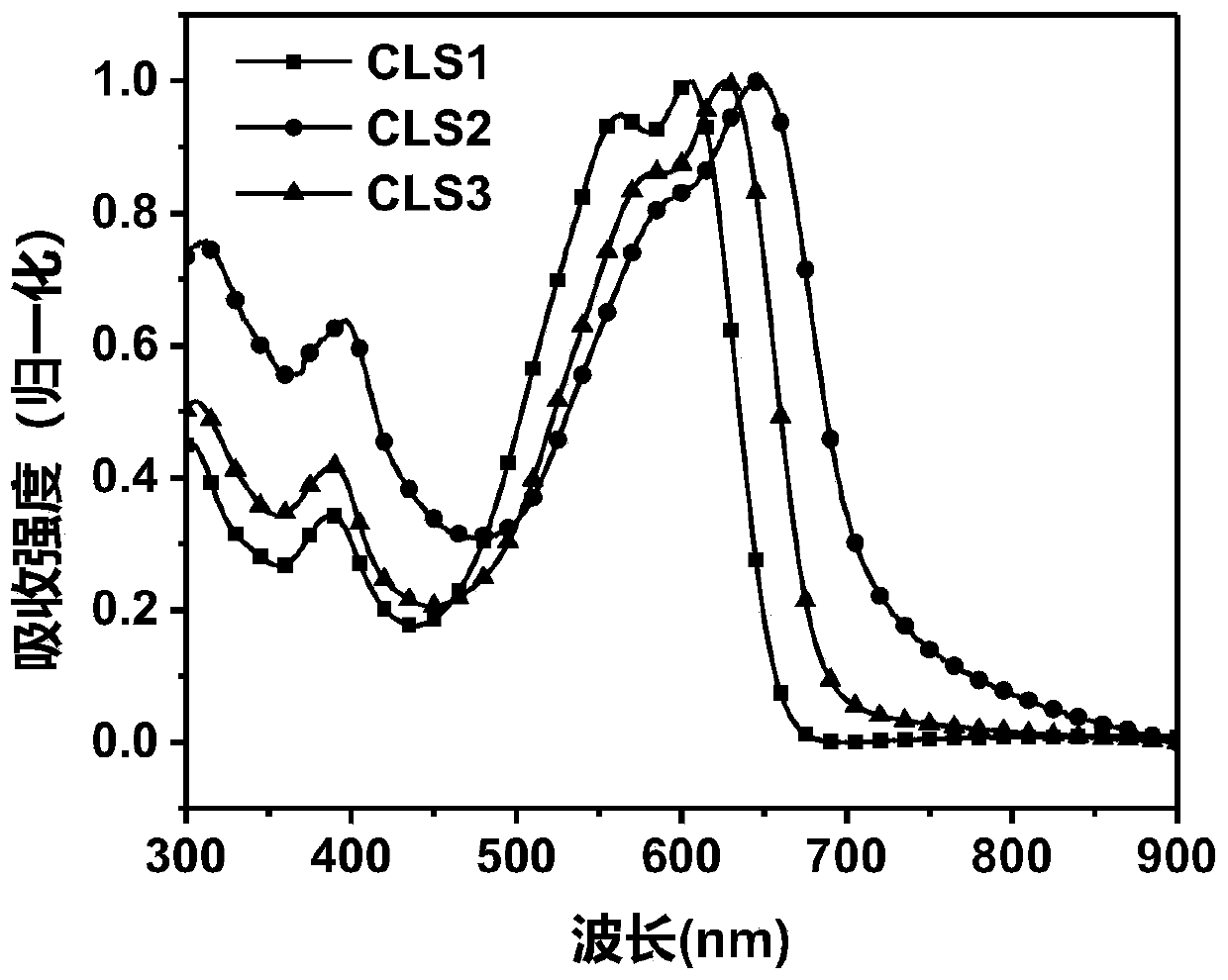
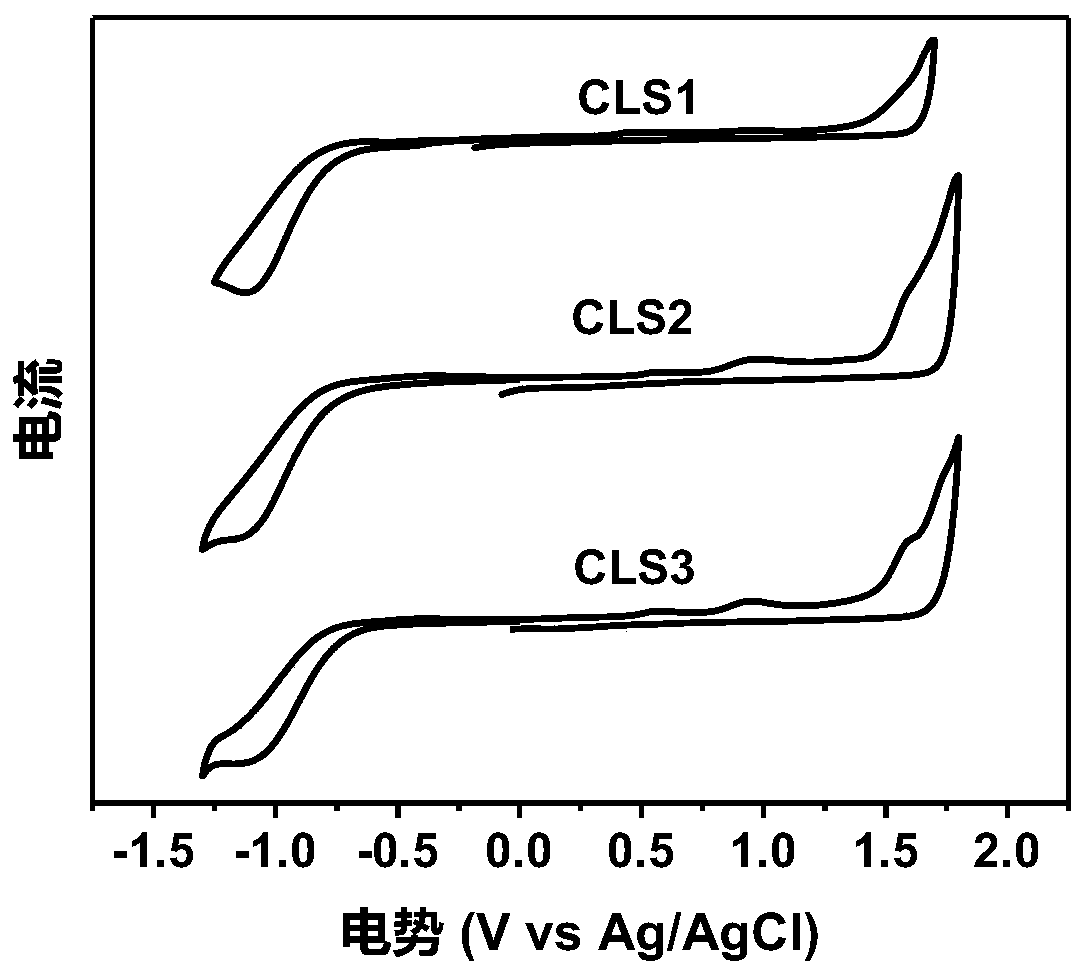
[0062] An A-π-D-π-A type organic small molecule CLS1 based on bis-indenopyrazine (IPY), with the electron-donating IPY unit as the central core D, thiophene as the π-conjugated bridge, 3-(dicyano Methylene) inden-1-one is the electron-withdrawing terminal A.
[0063] CLS1 has the general structural formula of Formula I:
[0064]
[0065] Wherein, R is n-octyl, and A is 3-(dicyanomethylene)inden-1-one.
[0066] The specific chemical structural formula of the small molecule CLS1 is:
[0067]
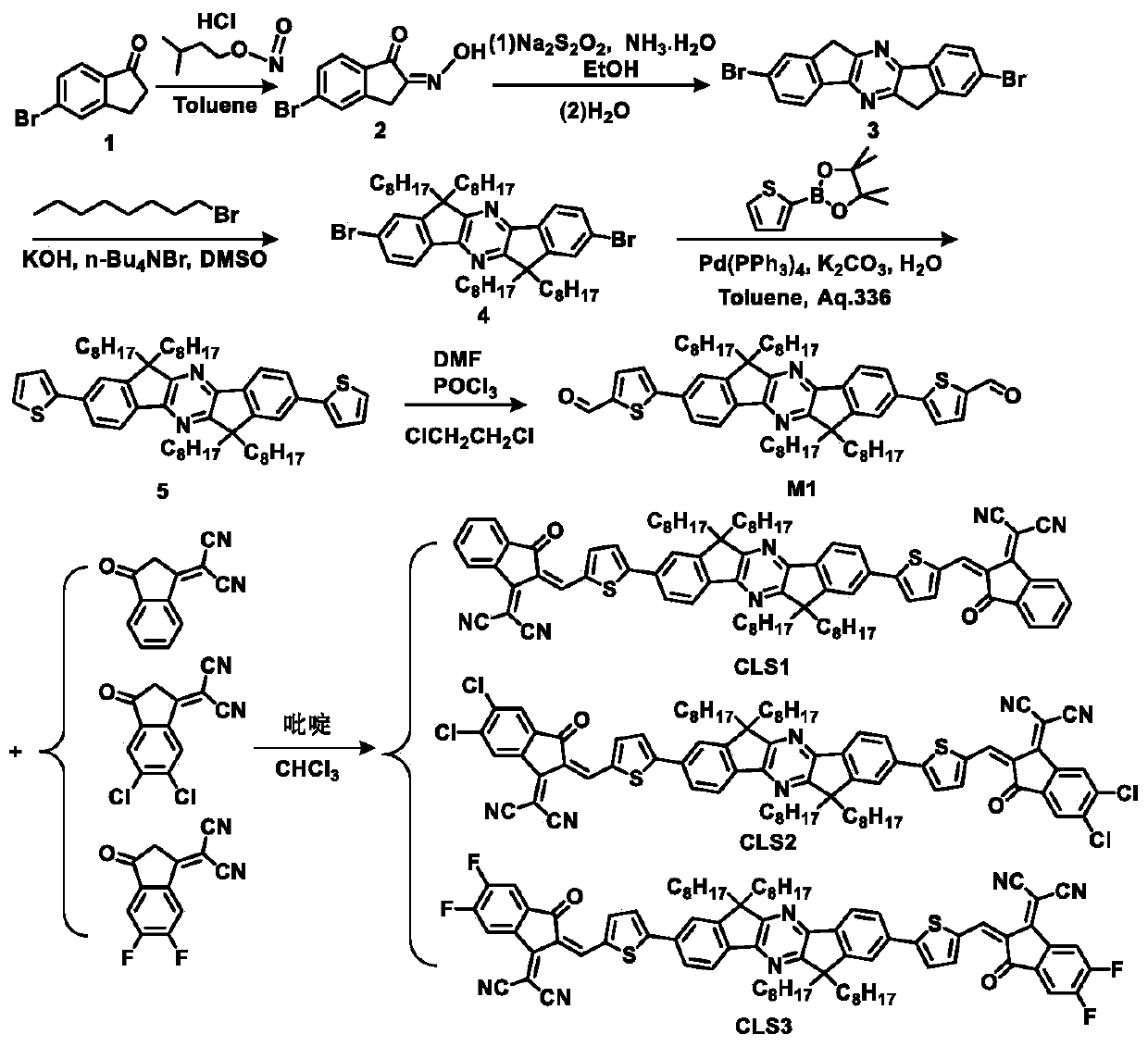
[0068] A preparation method of CLS1 of this embodiment, see the synthetic route figure 1 , the specific preparation method comprises the following steps:
[0069] (1) Synthesis of 5-bromo-2-oximino-2,3-dihydro-1-indanone (compound 2).
[0070] 1.1. Under argon atmosphere, add 5.00 g of 5-bromo-indanone (23.69 mmol) (compound 1) and 100 mL of toluene into a 250 mL three-necked flask.
[0071] 1.2. Bubble dry HCl gas into the reaction system for 5 minutes.
[0072] 1.3. Pass the ...
Embodiment 2
[0125] An A-π-D-π-A small molecule CLS2 based on the indenopyrazine (IPY) unit, with the electron-donating IPY unit as the central core D, thiophene as the π-conjugated bridge, and 5,6-di Chloro-3-(dicyanomethylene)inden-1-one is the electron-withdrawing terminal A.
[0126] CLS2 has the general structural formula of Formula I:
[0127]
[0128] Among them, R is n-octyl, A is 5,6-dichloro-3-(dicyanomethylene)inden-1-one, and the specific chemical structure formula of the small molecule CLS2 is as follows:
[0129]
[0130] A preparation method of CLS2 of this embodiment, see the synthetic route figure 1 , the specific preparation method comprises the following steps:
[0131] (1) Intermediate M1 5,5'-(6,6,12,12-tetraoctyl-6,12-dihydroindeno[1,2-b:1',2'-e]pyridine Synthesis of oxazine-2,8-diyl)bis(thiophene-2-carbaldehyde): same as Example 1.
[0132] (2) Synthesis of CLS2: under argon atmosphere, in a 100mL three-neck flask, add 0.60g of intermediate M1 (0.65mmol), 0...
Embodiment 3
[0145] An A-π-D-π-A type small molecule CLS3 based on an IPY unit: with the electron-donating IPY unit as the central core D, thiophene as the π-conjugated bridge, 5,6-difluoro-3-(dicyano Methylene) inden-1-one is the electron-withdrawing terminal A.
[0146] CLS3 has the general structural formula of I formula:
[0147]
[0148] Wherein, R is n-octyl, A is 5,6-difluoro-3-(dicyanomethylene)inden-1-one, and the specific chemical structural formula of the small molecule CLS3 is as follows:
[0149]
[0150] A preparation method of CLS3 of this embodiment, see the synthetic route figure 1 , the specific preparation method comprises the following steps:
[0151] (1) Intermediate M1 5,5'-(6,6,12,12-tetraoctyl-6,12-dihydroindeno[1,2-b:1',2'-e]pyridine Synthesis of oxazine-2,8-diyl)bis(thiophene-2-carbaldehyde): same as Example 1.
[0152] (2) Synthesis of CLS3: Add 0.67g of intermediate M1 (0.72mmol) and 0.66g of 5,6-difluoro-3-(dicyanomethylene) into a 100mL three-neck fl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com