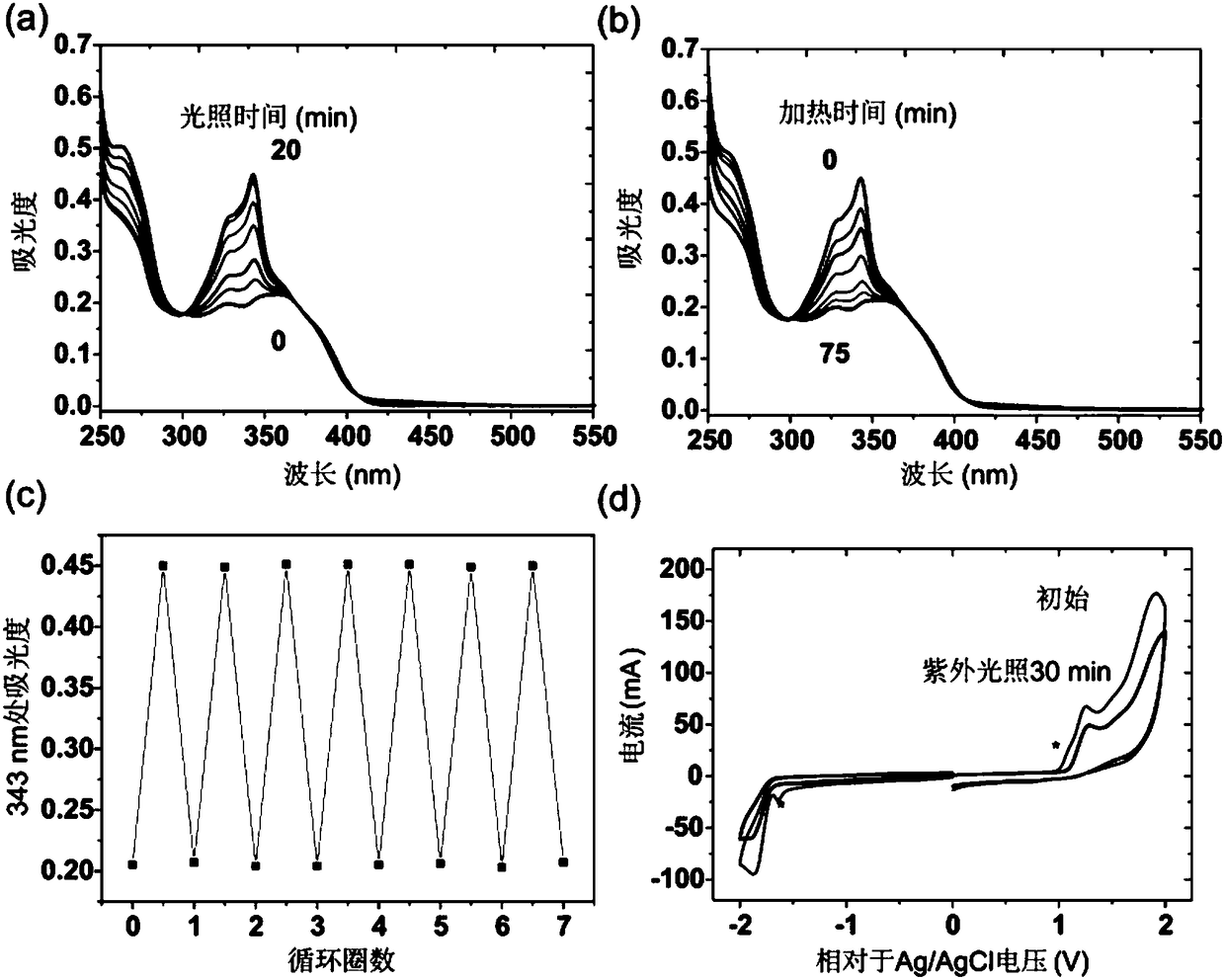
Photochromic compounds, preparation method thereof and photochromic products
A technology of photochromic compounds, applied in the preparation of carbon-based compounds, organic compounds, color-changing fluorescent materials, etc. With problems such as chirality, it can achieve the effects of large molecular energy level changes, large structural changes, and large dipole changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
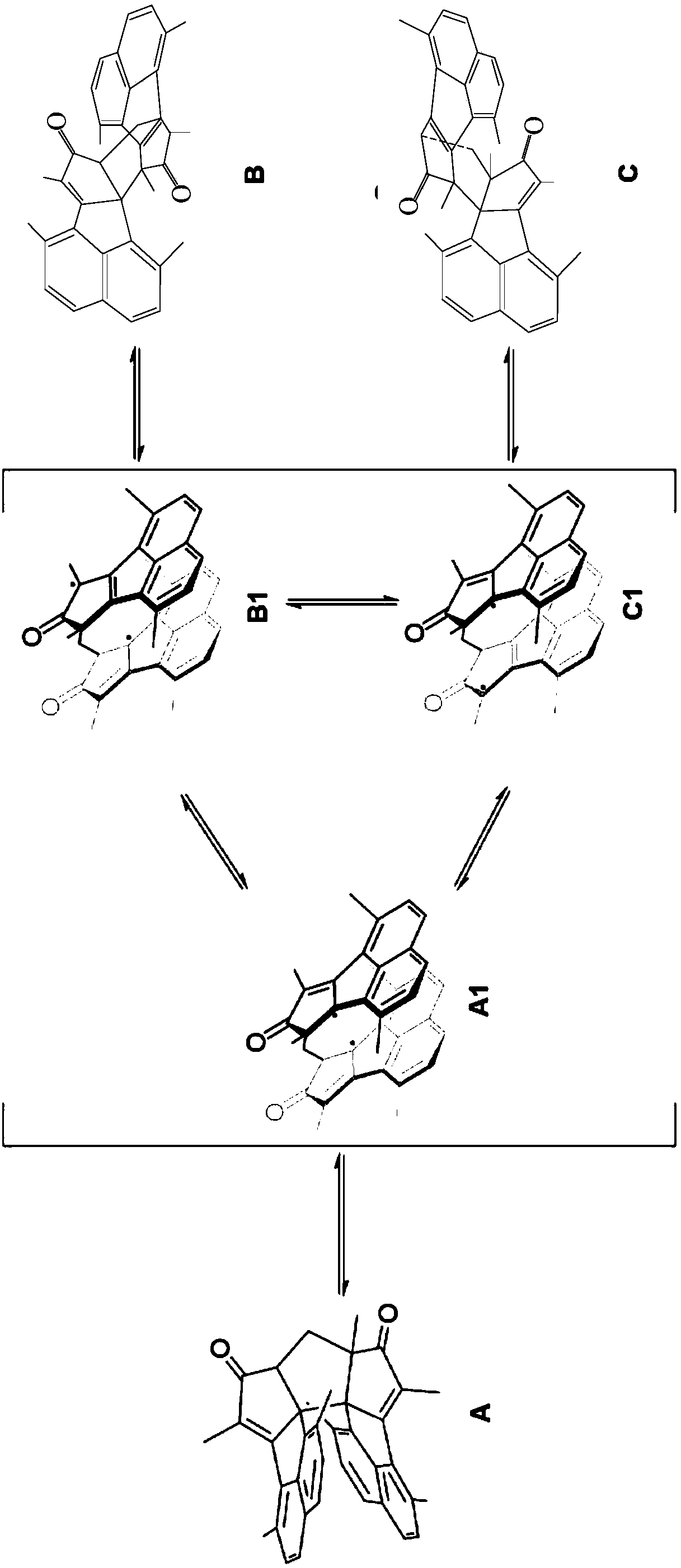
[0052] The present invention also provides a preparation method of the photochromic compound, comprising:
[0053] The compound M shown in the general formula M and the compound N shown in the general formula N are heated to reflux in an alkaline solution to obtain the photochromic compound I shown in the general formula I-I and the compound N shown in the general formula I - the photochromic compound II described in II:
[0054]
[0055] The preparation method of the photochromic compound can be completed in one pot, hereinafter referred to as one pot method. The preparation method has simple process and easy operation. The following is the synthetic mechanism of the photochromic compound:
[0056]
[0057] It is worth noting that the inventors found in the process of preparing the photochromic compound that when R 2 When it is a hydrogen atom, the compound of exo form cannot be obtained by the above-mentioned preparation method. The reason is that when the naphthale...
Embodiment 1
[0093] Compound 1 (1.5 g, 7.1 mmol), 3-pentanone (2.46 g, 28.6 mmol) and 15 mL of methanol were added to a round bottom flask. KOH (3.18g, 56.7mmol) was dissolved in 5mL of methanol, and slowly dropped into the above dispersion, then heated to reflux and reacted for 24h. After the reaction was completed, a solid was obtained by filtration, and the solid was washed 3 times with methanol. The crude product was separated by column chromatography and recrystallized with dichloromethane / methanol to obtain 853 mg of a light yellow solid, which was compound A, and the yield of compound A was 57%.
[0094] The synthetic route of described compound A is:
[0095]
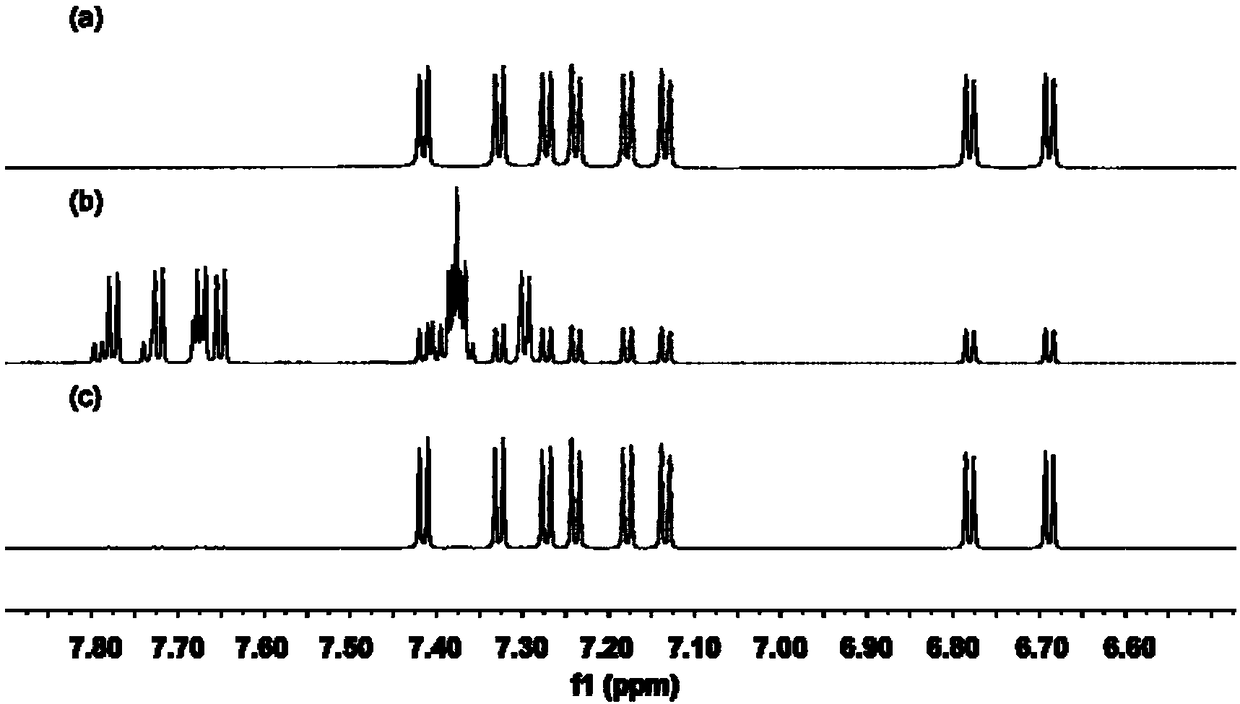
[0096] The measurement result of compound A nuclear magnetic resonance (NMR) and high-resolution mass spectrum is:
[0097] 1 H NMR (500MHz, CD 2 Cl 2 )δ7.42(d, J=8.3Hz, 1H), 7.33(d, J=8.3Hz, 1H), 7.27(d, J=8.2Hz, 1H), 7.24(d, J=8.3Hz, 1H) ,7.18(d,J=8.3Hz,1H),7.13(d,J=8.3Hz,1H),6.78(d,J=8.2Hz,1H),6.69(d,J=8.3Hz,1H),...
Embodiment 2
[0103]Add compound 1 (1g, 4.76mmol) and chloroform (30ml) into a 100ml three-necked flask. Liquid bromine (1.13ml, 22.0mmol) and chloroform (15ml) were added to the dropping funnel. At room temperature, the liquid bromine solution was slowly added dropwise. After the drop, the reaction solution was heated to 85° C. for 3 hours, and then the temperature was lowered. Anhydrous sodium sulfite solution was added in an ice bath to quench excess bromine. The reaction solution was diluted with dichloromethane, washed three times with water, dried over anhydrous sodium sulfate, filtered and the solvent was removed. The crude product was recrystallized from chloroform and methanol to obtain 1.02 g of a yellow solid, which was compound 3, and the yield of compound 3 was 73%.
[0104] Weigh compound 3 (1.00g, 3.46mmol) into a round bottom flask, add 3-pentanone (1.46ml, 14.0mmol) and methanol (14.6ml). Slowly add a solution of potassium hydroxide (1.5g, 27mmol) in methanol (7.4ml) to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com