A'-pi-A-pi-A' type organic small molecule and preparation method and application thereof
A small molecule, organic technology for use in solar cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
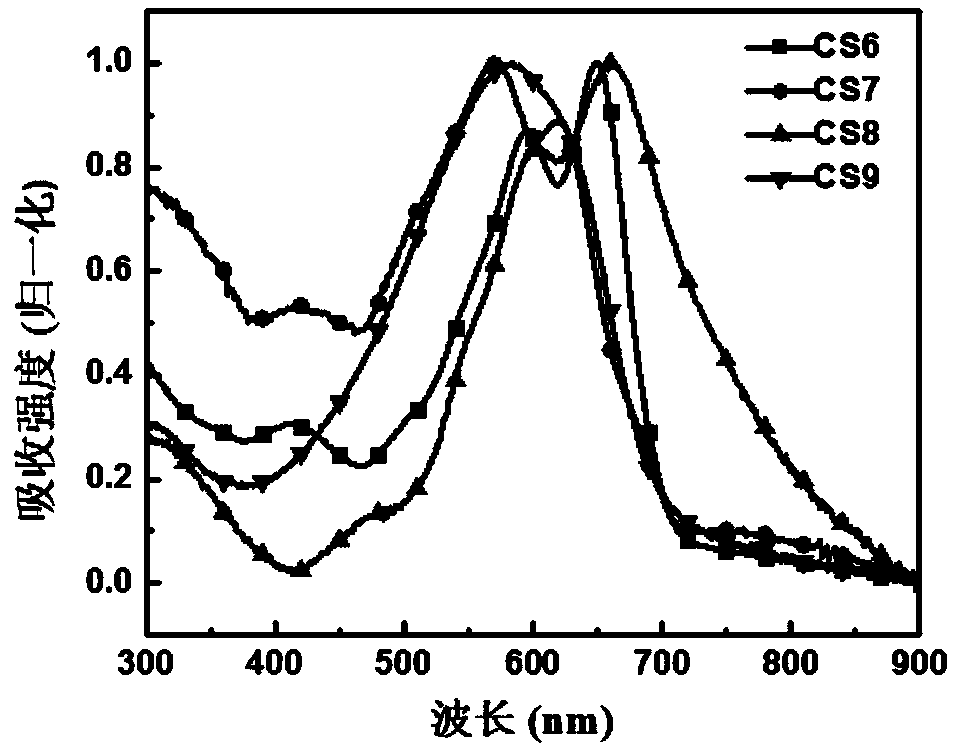
Image
Examples
Embodiment 1
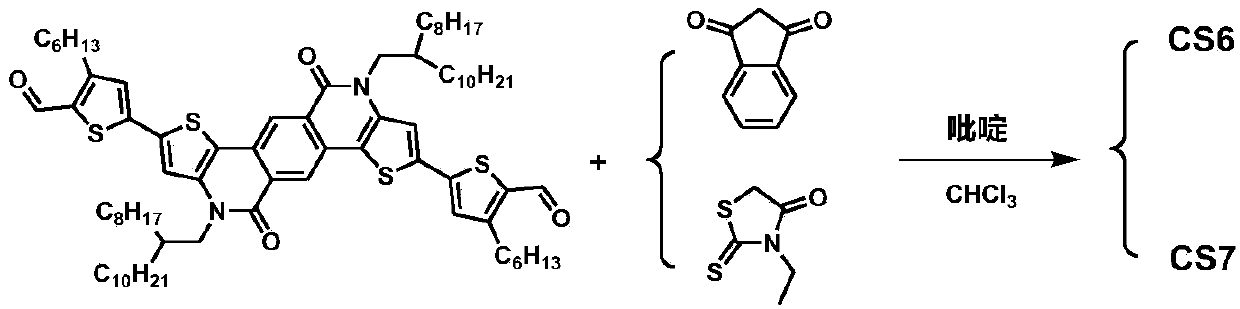
[0092] An A′-π-A-π-A′-type small molecule CS6 based on TPTI units, with a weakly electron-withdrawing TPTI unit as the central core A, 3-hexylthiophene as the π-conjugated bridge, and 1,3-indane The diketone is the electron-withdrawing terminal A'.
[0093] CS6 has the general structural formula of formula I:
[0094]
[0095] Wherein, R is 2-octyldodecyl, R 1 is n-hexyl, A' is 1,3-indandione.
[0096] The specific chemical structural formula of CS6 is:
[0097]
[0098] A preparation method of CS6 of this embodiment, see the synthetic route figure 1 , the specific preparation method comprises the following steps:
[0099] (1) 2,5-Dibromo-N 1 ,N 4 -Bis(2-octyldodecyl)-N 1 ,N 4 - Synthesis of bis(thiophen-3-yl)terephthalamide (compound 3).
[0100] 1.1. Under argon atmosphere, in a 250mL three-necked flask, add 11.6g (30.56mmol) N-(2-octyldodecyl)thiophene-3-amine (compound 1), 5mL triethylamine and 40mL dry of dichloromethane.
[0101] 1.2. Dissolve 5 g (13.89...
Embodiment 2
[0159] An A′-π-A-π-A′-type small molecule CS7 based on TPTI units, with a weakly electron-withdrawing TPTI unit as the central core A, 3-hexylthiophene as the π-conjugated bridge, and 3-ethylrhodan Ning is the electron-withdrawing terminal A'.
[0160] CS7 has the general structural formula of formula I:
[0161]
[0162] Wherein, R is 2-octyldodecyl, R 1 is n-hexyl, A' is 3-ethyl rhodanine, and the specific chemical structural formula is as follows:
[0163]
[0164] A preparation method of CS7 of this embodiment, see the synthetic route figure 1 , the specific preparation method comprises the following steps:
[0165] (1) Intermediate M1 (5,5'-(4,10-bis(2-octyldodecyl)-5,11-dioxo4,5,10,11tetrahydrothiophene[2', Synthesis of 3':5,6]pyrido[3,4-g]thieno[3,2-c]isoquinoline-2,8-diyl)bis(3-hexylthiophene-2-carbaldehyde)) : Same as Example 1.
[0166] (2) Synthesis of CS7: as a raw material, under argon atmosphere, add 0.82g of intermediate M1 (0.63mmol) and 0.40g of 3-...
Embodiment 3
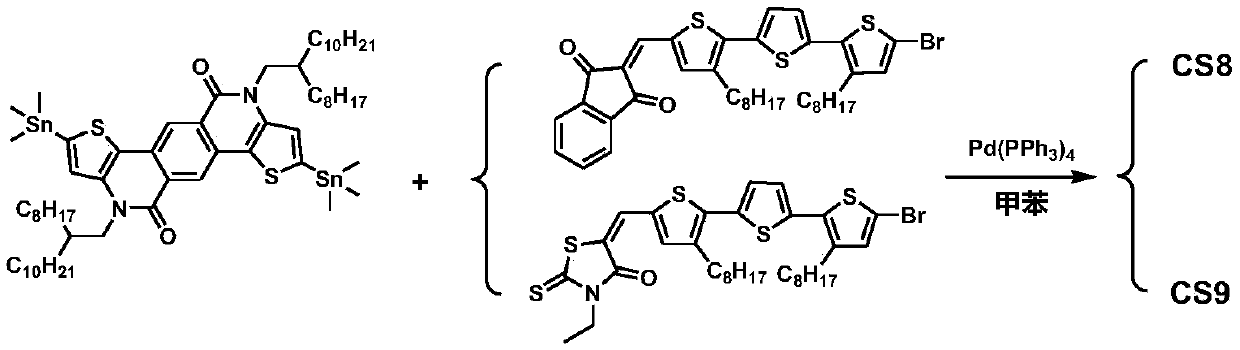
[0179] A TPTI unit-based A′-π-A-π-A′-type small molecule CS8: a weakly electron-withdrawing TPTI unit as the central core A, 3,3″-dioctyl-(2,2′:5 ',2″-trithiophene) is the π-conjugated bridge, and 1,3-indandione is the electron-withdrawing terminal A'.
[0180] CS8 has the general structural formula of formula II:
[0181]
[0182] Wherein, R is 2-octyldodecyl, R 1 is n-octyl, A' is 1,3-indanedione, and the specific chemical structural formula of the small molecule CS8 is as follows:
[0183]
[0184] A preparation method of CS8 of this embodiment, see the synthetic route figure 2 , the specific preparation method comprises the following steps:
[0185] (1) Compound 4(4,10-bis(2-octyldodecyl)-4,10-dihydrothieno[2',3':5,6]pyrido[3,4-g] Synthesis of thieno[3,2-c]isoquinoline-5,11-dione): same as Example 1.
[0186] (2) 4,10-bis(2-octyldodecyl)-2,8-bis(trimethylstannyl)-4,10-dihydrothieno[2',3':5, 6] Synthesis of pyrido[3,4-g]thieno[3,2-c]isoquinoline-5,11-dione (int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com