A focus in situ drug controlled release system and preparation method thereof
A technology of controlled release and drug, applied in the field of medical devices, can solve the problems of limited drug loading and insufficient depth of infiltration of lesion cells, and achieve the effects of reducing recurrence, increasing drug loading, and improving cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
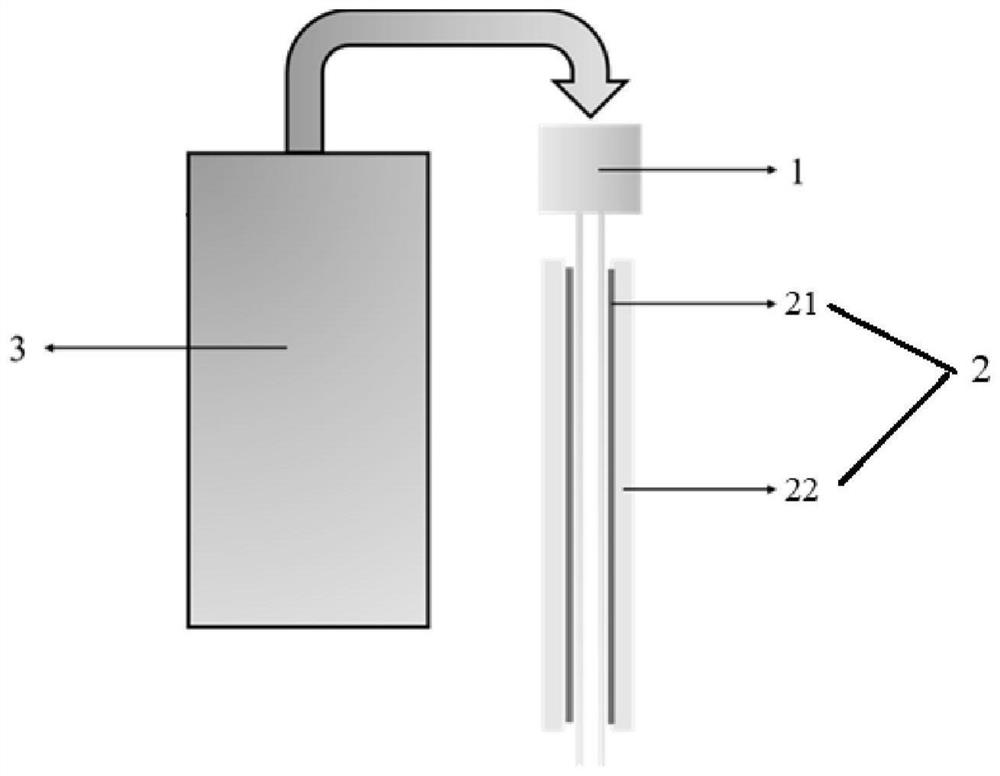
[0096] This embodiment provides a system for controlled drug release in situ at the lesion, which includes a 26G electrospinning needle, an inner polyethylene glycol (molecular weight 6000Da) coating covering the outer surface of the needle, and an outer coating covering the outer surface of the inner coating. Copolymer of polylactic acid-co-glycolic acid (molecular weight 60000Da) coating and a medical syringe connected with a needle.
[0097] Its preparation method is:
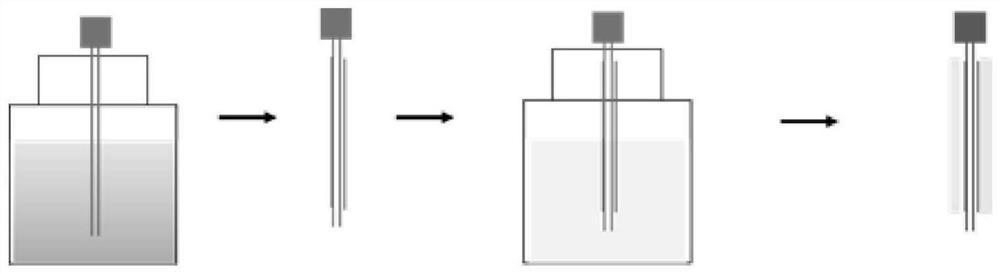
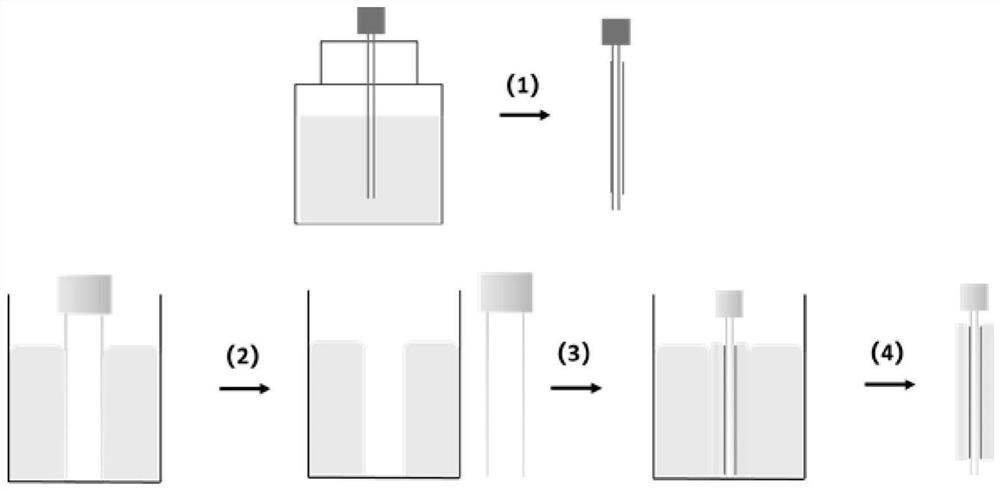
[0098] (1) Dissolve polylactic acid-glycolic acid copolymer and N,N-dimethylformamide under heating and stirring at 35°C, and the solution concentration is 80%; dissolve polyethylene glycol and ethanol at 35°C Heat and stir to dissolve, the solution concentration is 85%;
[0099] (2) Use a 26G electrospinning needle to dip the polyethylene glycol solution into glue at 25°C, and then vacuum-dry it at 25°C for 2 hours;
[0100] (3) The 26G spinning needle with the inner layer coating is dipped in the polylac...
Embodiment 2
[0107] This embodiment provides a system for controlled drug release in situ at the lesion, which includes a 26G electrospinning needle, an inner polyethylene glycol (molecular weight 6000Da) coating covering the outer surface of the needle, and an outer coating covering the outer surface of the inner coating. Copolymer of polylactic acid-co-glycolic acid (molecular weight 60000Da) coating and a medical syringe connected with a needle.
[0108] Its preparation method is consistent with embodiment 1.
[0109] Carry out drug release experiment to the obtained product, and draw release curve, method is as follows:
[0110] (1) Load the drug 5-fluorouracil in the hollow part of the product, and the mass of 5-fluorouracil is 50% of the total mass of the inner coating and the outer coating, and the upper and lower ends are capped with polylactic acid-glycolic acid copolymer, and then its Put it into a centrifuge tube containing 10 mL of fresh Tris-HCl solution;
[0111] (2) Then p...
Embodiment 3
[0115] This embodiment provides a system for controlled drug release in situ at the lesion, which includes a 26G electrospinning needle, an inner polyethylene glycol (molecular weight 6000Da) coating covering the outer surface of the needle, and an outer coating covering the outer surface of the inner coating. Copolymer of polylactic acid-co-glycolic acid (molecular weight 60000Da) coating and a medical syringe connected with a needle.
[0116] Its preparation method is consistent with embodiment 1.
[0117] Carry out drug release experiment to the obtained product, and draw release curve, method is as follows:
[0118] (1) The drug capecitabine is loaded into the hollow part of the product, the quality of capecitabine is 50% of the total mass of the inner coating and the outer coating, and the upper and lower ends are capped with polylactic acid-glycolic acid copolymer, and the latter Put it into a centrifuge tube containing 10 mL of fresh PBS solution;
[0119] (2) Then pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com