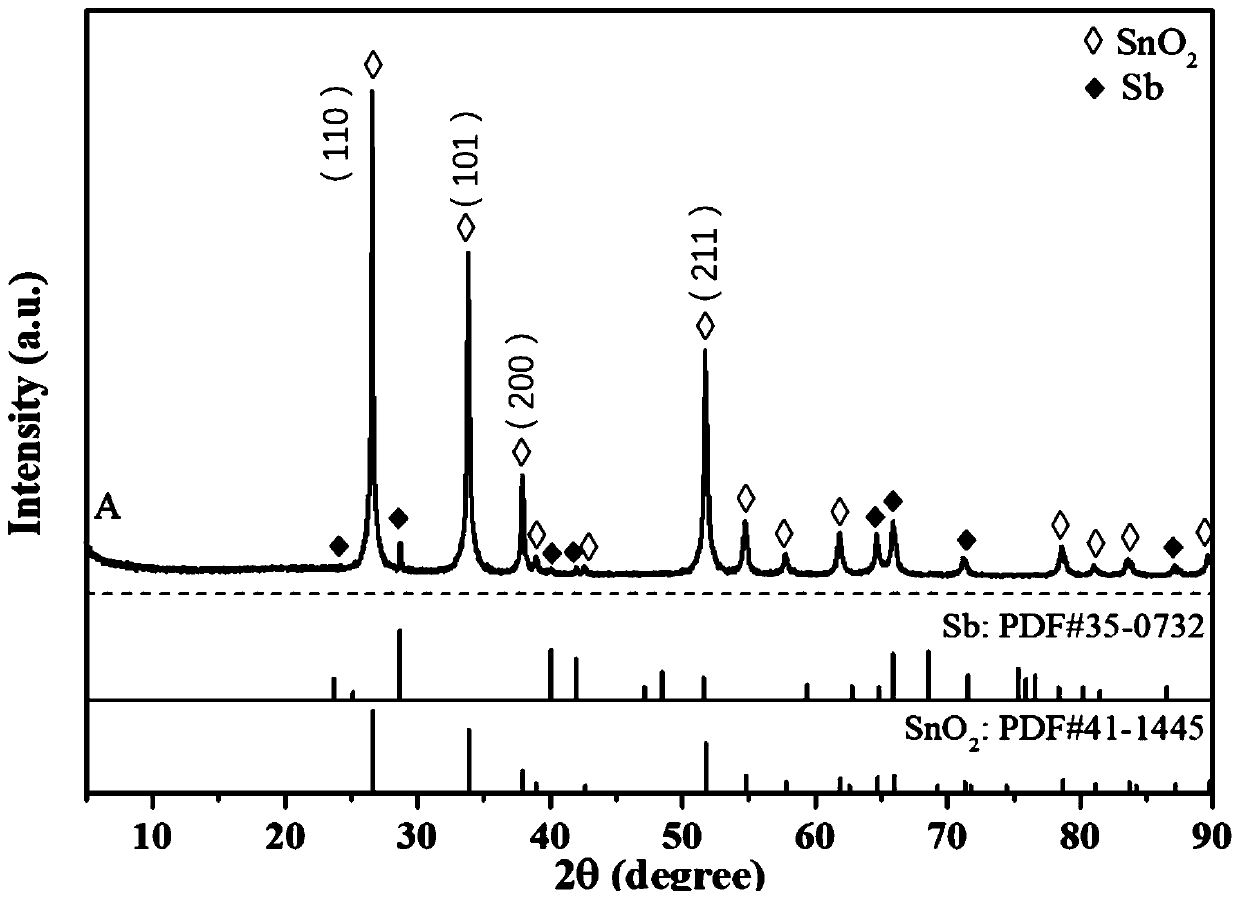
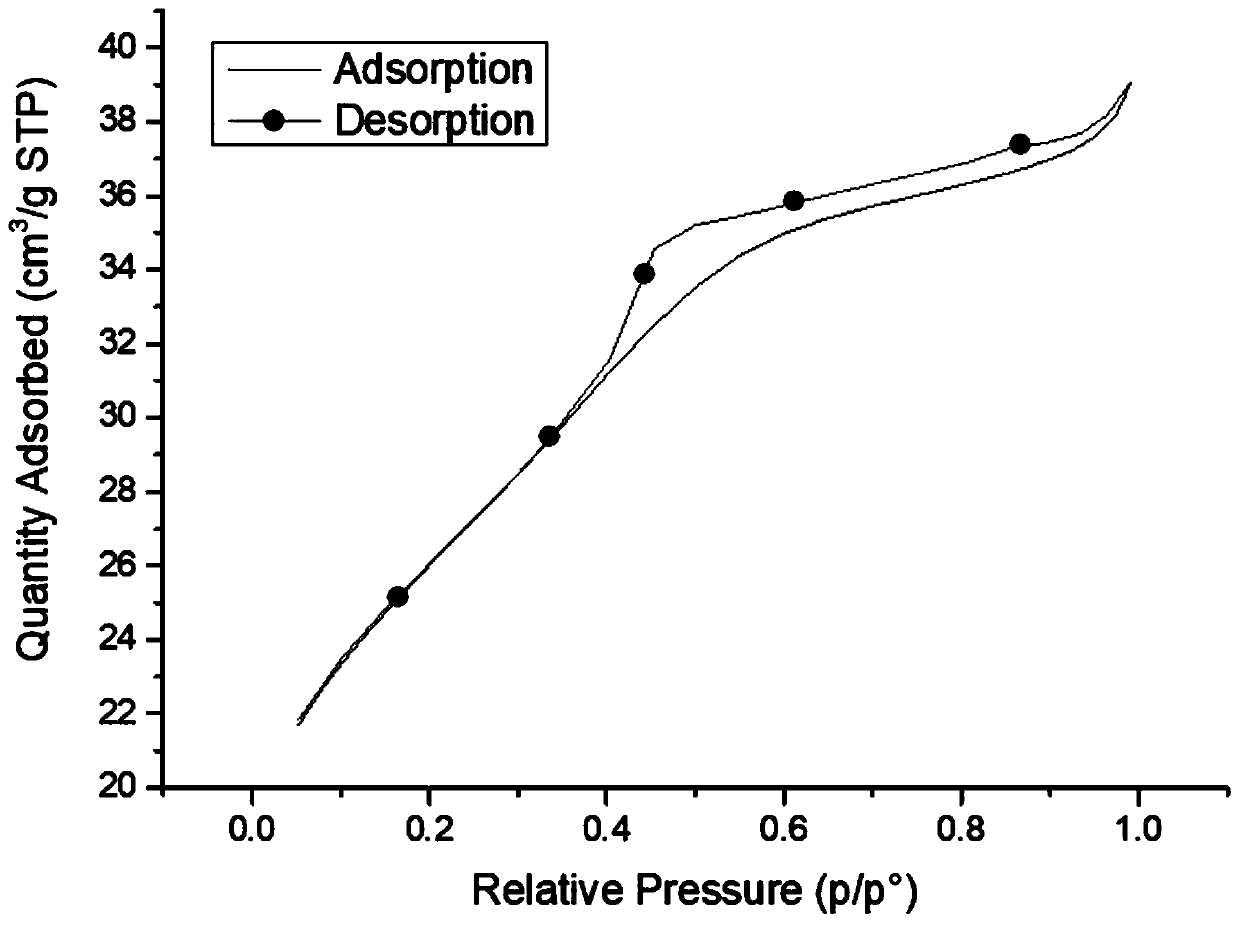
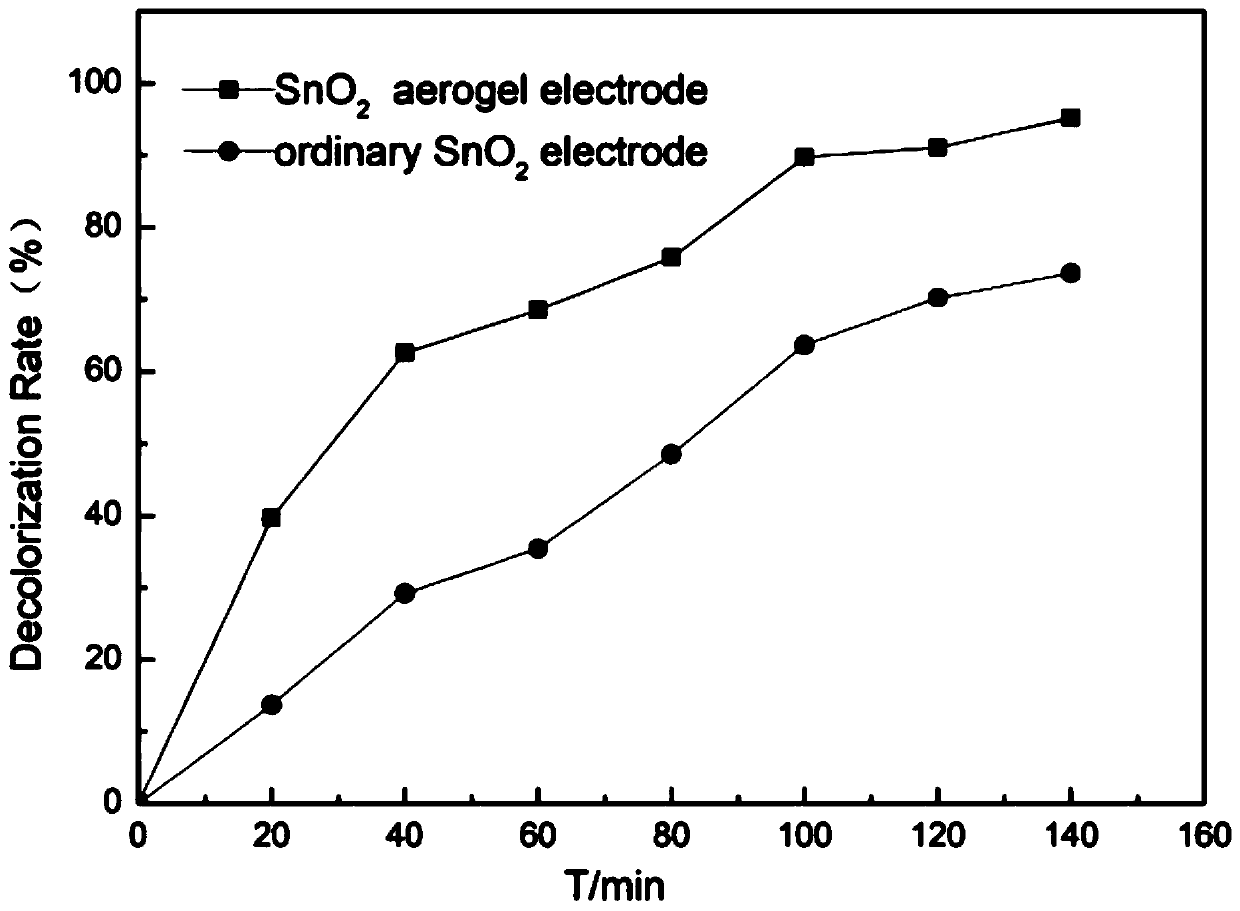
Preparation method of antimony-doped stannic oxide aerogel-titanium electrode
A tin dioxide and aerogel technology, applied in the field of electrodes, can solve the problems of low electrode specific surface area, limited electrochemical area, and few catalytic active sites, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (11) Grinding the surface of the titanium sheet with sandpaper, soaking the polished titanium sheet into a NaOH solution with an initial temperature of 90° C. and a concentration of 9% to boil for 50 minutes to remove oil stains on the surface of the titanium sheet;
[0032] (12) put the titanium sheet with the surface oil stain removed into the concentration of 9% oxalic acid solution at 55° C. The titanium sheet is immersed in the ethanol solution and stored for later use;
[0033] (13) to SnCl 4 ·5H 2 Add deionized water to O and stir until SnCl 4 ·5H 2 Obtain colorless and transparent liquid A after O is completely dissolved; to SbCl 3 Concentrated hydrochloric acid was added dropwise, and stirred until SbCl 3 Obtain colorless and transparent liquid B after complete dissolution;
[0034] (14) Mix the liquid A and the liquid B to obtain the mixed liquid C, if the mixed liquid C becomes turbid, add concentrated hydrochloric acid until the mixed liquid C is color...
Embodiment 2
[0040] (21) Grinding the surface of the titanium sheet with sandpaper, soaking the polished titanium sheet into an initial temperature of 90° C. and boiling in a 10% NaOH solution for 60 minutes to remove oil stains on the surface of the titanium sheet;
[0041](22) Put the titanium sheet that has removed surface oil stains into a 10% oxalic acid solution at 60°C and etch for 2 hours, so that the surface of the etched titanium sheet appears gray, uniform and rough, and the etched titanium sheet The slices were immersed in ethanol solution and stored for later use;
[0042] (23) to SnCl 4 ·5H 2 Add deionized water to O and stir until SnCl 4 ·5H 2 Obtain colorless and transparent liquid A after O is completely dissolved; to SbCl 3 Concentrated hydrochloric acid was added dropwise, and stirred until SbCl 3 Obtain colorless and transparent liquid B after complete dissolution;
[0043] (24) Mix the liquid A and the liquid B to obtain a mixed liquid C, if the mixed liquid C be...
Embodiment 3
[0049] (31) Grinding the surface of the titanium sheet with sandpaper, infiltrating the polished titanium sheet into an initial temperature of 90° C. and boiling in an 11% NaOH solution for 70 minutes to remove oil stains on the surface of the titanium sheet;
[0050] (32) Put the titanium sheet from which surface oil stains have been removed into an oxalic acid solution with a concentration of 11% at 65° C. The titanium sheet is immersed in the ethanol solution and stored for later use;
[0051] (33) to SnCl 4 ·5H 2 Add deionized water to O and stir until SnCl 4 ·5H 2 Obtain colorless and transparent liquid A after O is completely dissolved; to SbCl 3 Concentrated hydrochloric acid was added dropwise, and stirred until SbCl 3 Obtain colorless and transparent liquid B after complete dissolution;
[0052] (34) Mixing the liquid A and the liquid B to obtain the mixed liquid C, if the mixed liquid C becomes turbid, add concentrated hydrochloric acid until the mixed liquid C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com