Green phthalocyanine compound and preparation method thereof
A technology of compound and phthalocyanine, applied in the field of green phthalocyanine compound and its preparation, can solve problems such as not suitable for large-scale industrial application, insufficient brightness and contrast, insufficient dispersion, etc., achieve low environmental load, avoid light scattering, high contrast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
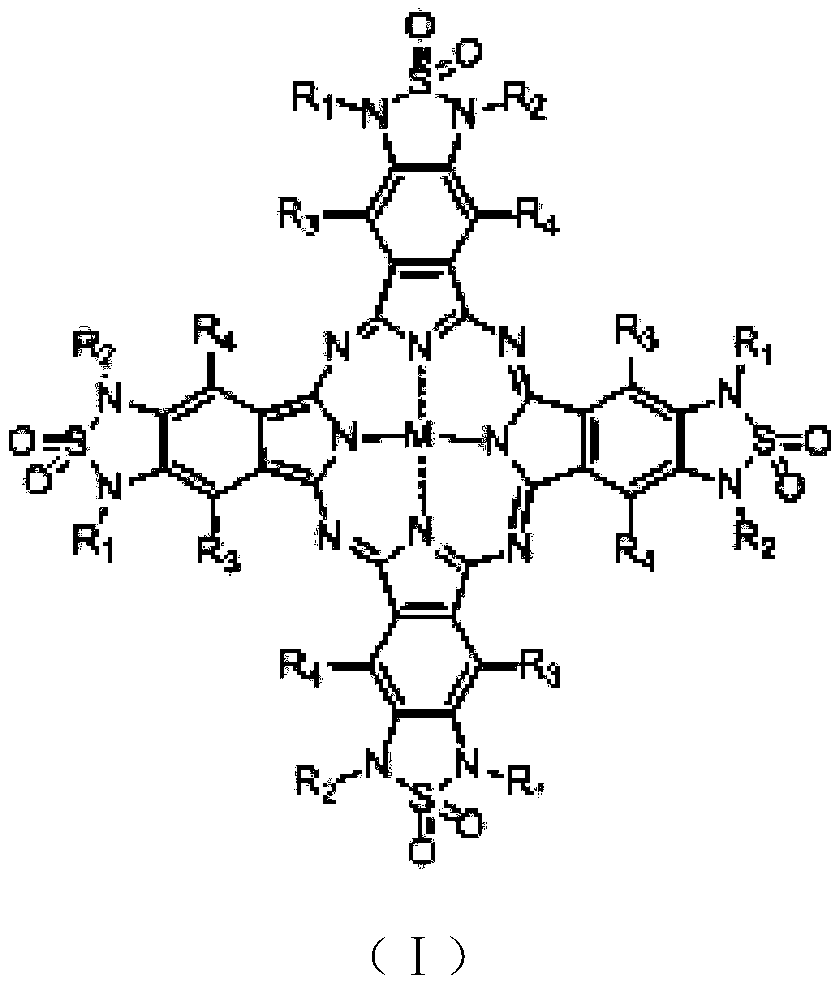
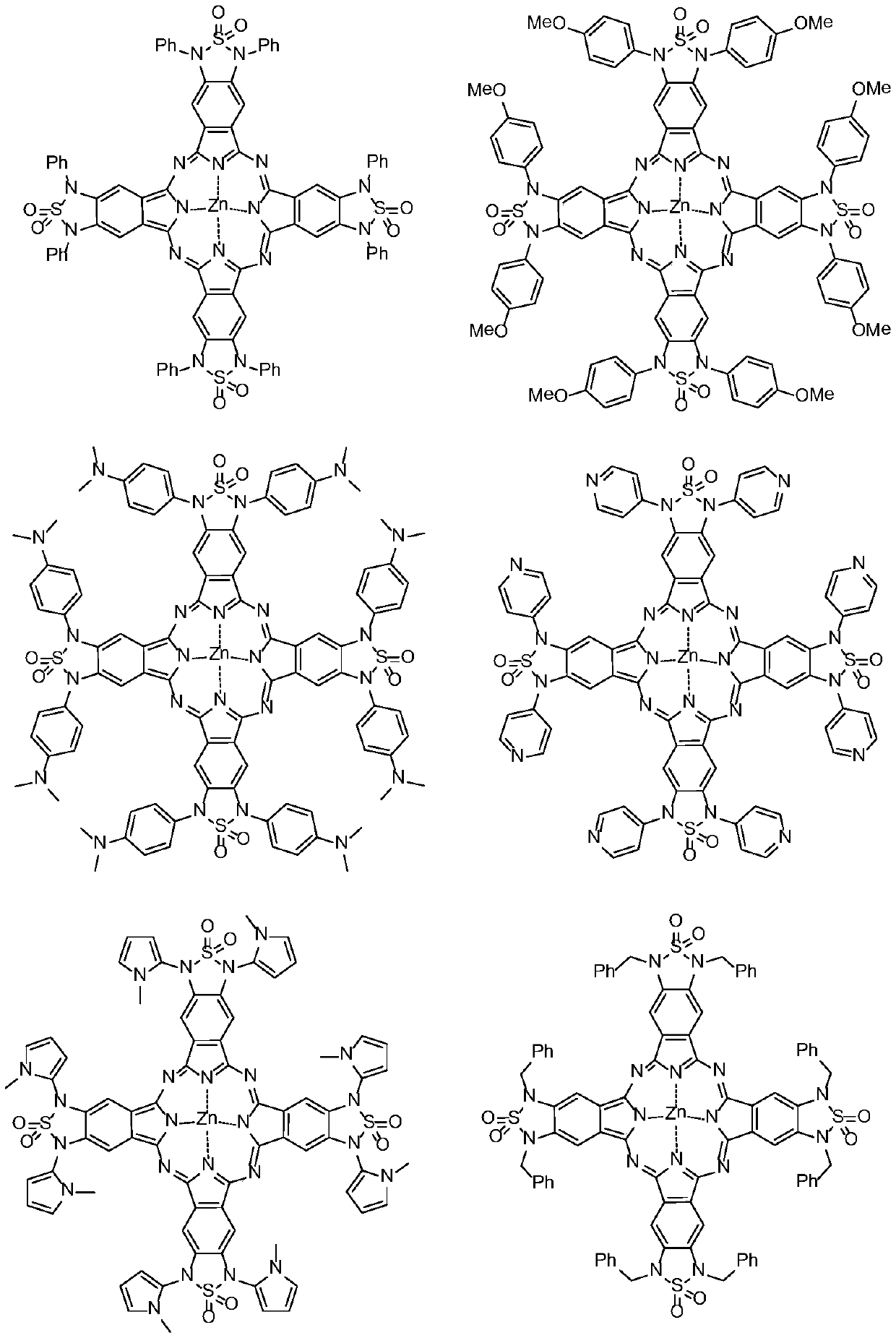
[0043] This example provides a preferred green phthalocyanine compound and its preparation method. The compound is represented by the formula ZnPc2-1, and its reaction precursor is represented by D-6. Concrete preparation method and synthetic route are as follows:
[0044] 1. Synthesis of intermediate product D-2
[0045] (1) Under the protection of nitrogen, add 1eq of raw material D (38.18g, 0.15mol), 1.05eq of aniline (15.12g, 0.1575mol) and 220ml of DMF into the flask, and stir evenly. 1.1eq potassium carbonate (23.03g, 0.165mol) was dissolved in 40ml of water, and added to the reaction system under nitrogen protection. The temperature was raised to 50° C., and the reaction was carried out under nitrogen protection for 3 hours.
[0046] (2) The reaction solution was added to 1000 ml of water, a solid was precipitated, filtered, and the solid was washed with water until colorless to obtain an orange solid. After drying, 36.5 g of the product was obtained with a yield of...
Embodiment 2
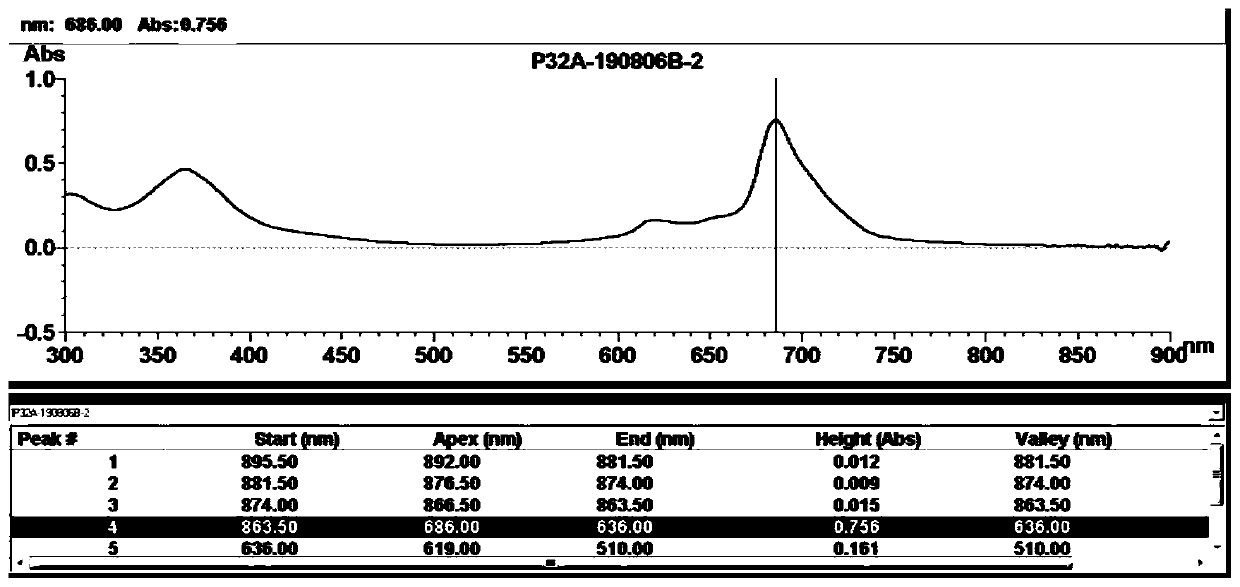
[0082] In this embodiment, the solubility and contrast of the phthalocyanine compound ZnPc2-1 in the first embodiment and the contrast of its derivatives are tested.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com