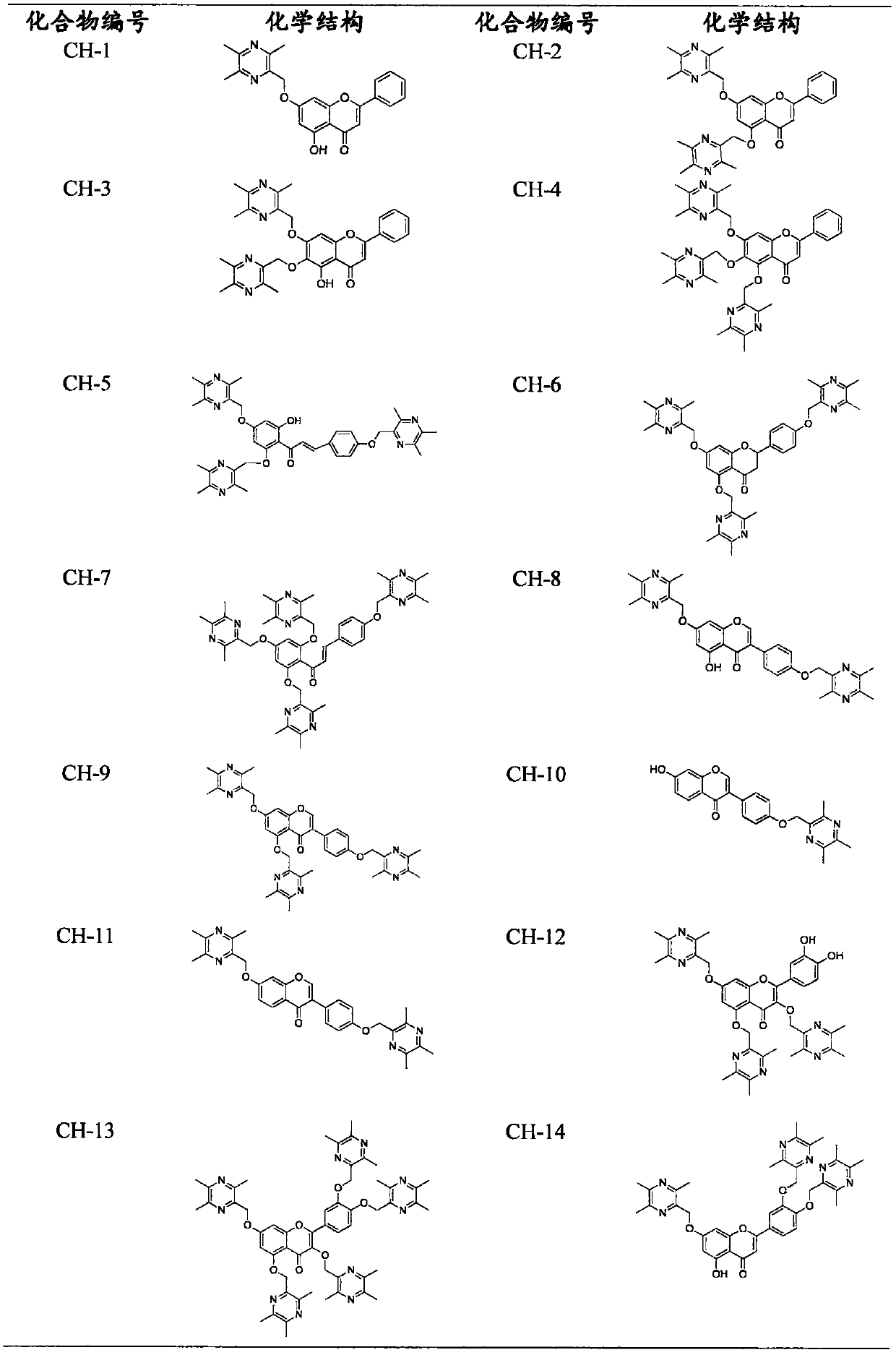
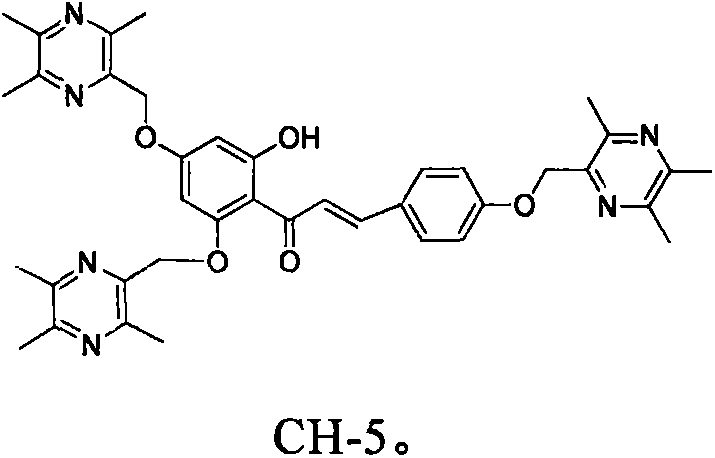
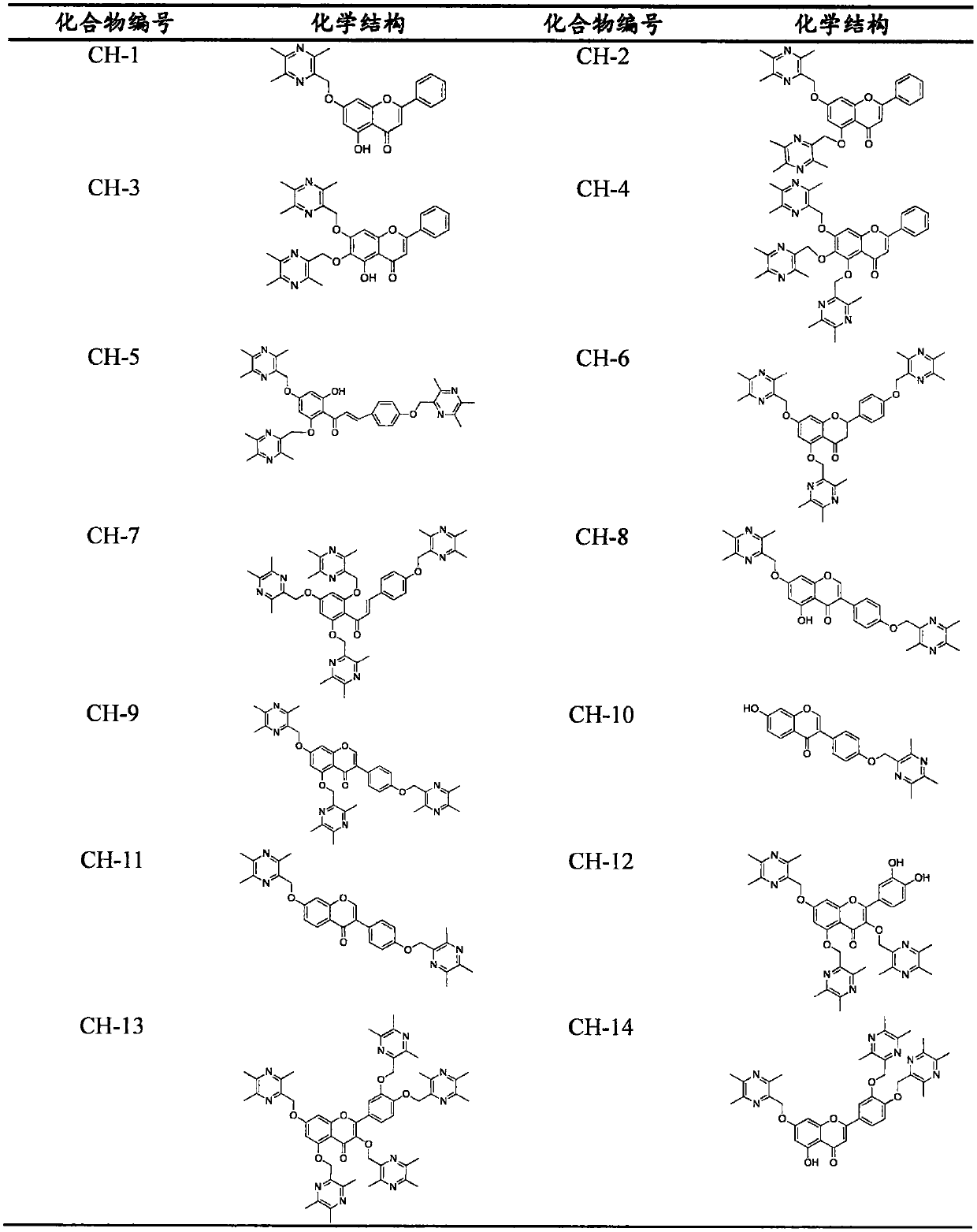
Flavone-ligustrazine compounds CH-X having selective anti-liver cancer effect, preparation method and applications thereof
A compound and anti-liver cancer technology, applied in medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc., can solve problems such as vascular side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] The preparation of embodiment 1 intermediate tetramethylpyrazine bromide (TMP-Br)
[0105] Weigh TMP·3H 2 An appropriate amount of O was stirred and dissolved in 2-3 times the amount of toluene, heated to reflux for 10-12 hours, and dehydrated in a water separator to obtain anhydrous TMP. Dissolve an appropriate amount of anhydrous TMP in CCl 4 Stir in medium to dissolve, and then add NBS according to the molar ratio TMP:NBS=1:0.7-0.9. Under the full irradiation of an incandescent lamp, heat and reflux for 10 hours. When the reaction is completed, the reaction liquid is purple-red, and the generated succinimide is suspended on it. Cool, filter, concentrate under reduced pressure in a water bath at 60-80°C and take it away. Excessive ligustrazine yields purple-red semi-oil TMP-Br, Yield: 70%.
Embodiment 2
[0106] Example 2 Preparation of Ligustrazine and Chrysin Derivatives (CH-1)
[0107] Weigh 0.50g of chrysin into a 100mL round bottom flask, put into TMP-Br at a molar ratio of 1:1, add an appropriate amount of anhydrous DMF to the reaction flask and stir to dissolve, then add an appropriate amount of K 2 CO 3 Finally, under the protection of nitrogen, the reaction bottle was placed in an oil bath at 75° C. for a heating reaction for 2 h (TLC followed the reaction). After the reaction is complete, cool and filter. The filtrate was heated in a water bath at 55 °C, and DMF was removed with a rotary evaporator. After reconstitution with dichloromethane, add silica gel to mix the sample, separate and purify on silica gel column, eluting with dichloromethane / acetone=10:1-3:1, improve the color development of potassium bismuth iodide, detect by TLC, and obtain white The solid object is compound CH-1. M.p.: 156.2-157.1℃, yield 58%. 1 H-NMR (CDCl 3 )(ppm): δ12.72(s, 1H), 7.88-7....
Embodiment 3
[0108] Example 3 Preparation of Ligustrazine and Chrysin Derivatives (CH-2)
[0109] Weigh 0.50g of chrysin into a 100mL round bottom flask, put into TMP-Br at a molar ratio of 1:2, add an appropriate amount of anhydrous DMF to the reaction flask and stir to dissolve, then add an appropriate amount of K 2 CO 3 Finally, under the protection of nitrogen, the reaction bottle was placed in an oil bath at 75° C. for a heating reaction for 2 h (TLC followed the reaction). After the reaction is complete, cool and filter. The filtrate was heated in a water bath at 55 °C, and DMF was removed with a rotary evaporator. After reconstitution with dichloromethane, add silica gel to mix the sample, separate and purify on silica gel column, eluting with dichloromethane / acetone=10:1-3:1, improve the color development of potassium bismuth iodide, detect by TLC, and obtain white The powdery solid target, namely compound CH-2. M.p.: 223.1-224.2℃, yield 60%. 1 H-NMR (CDCl 3 )(ppm) δ7.83-7.81...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com