Method for producing a hygroscopic alkali metal salt electrolyte solution
A technology of alkali metal salts and non-aqueous electrolytes, which is applied in the field of preparation of low-water-content electrolyte solutions, can solve problems such as time-consuming, environmental hazards, and expensive, and achieve the effects of reducing solvent consumption and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
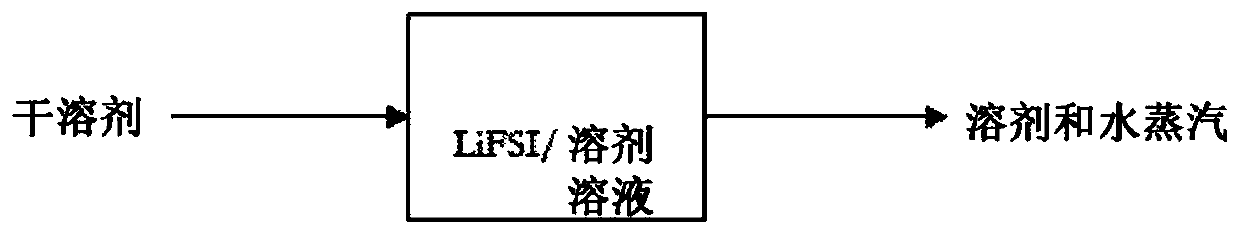
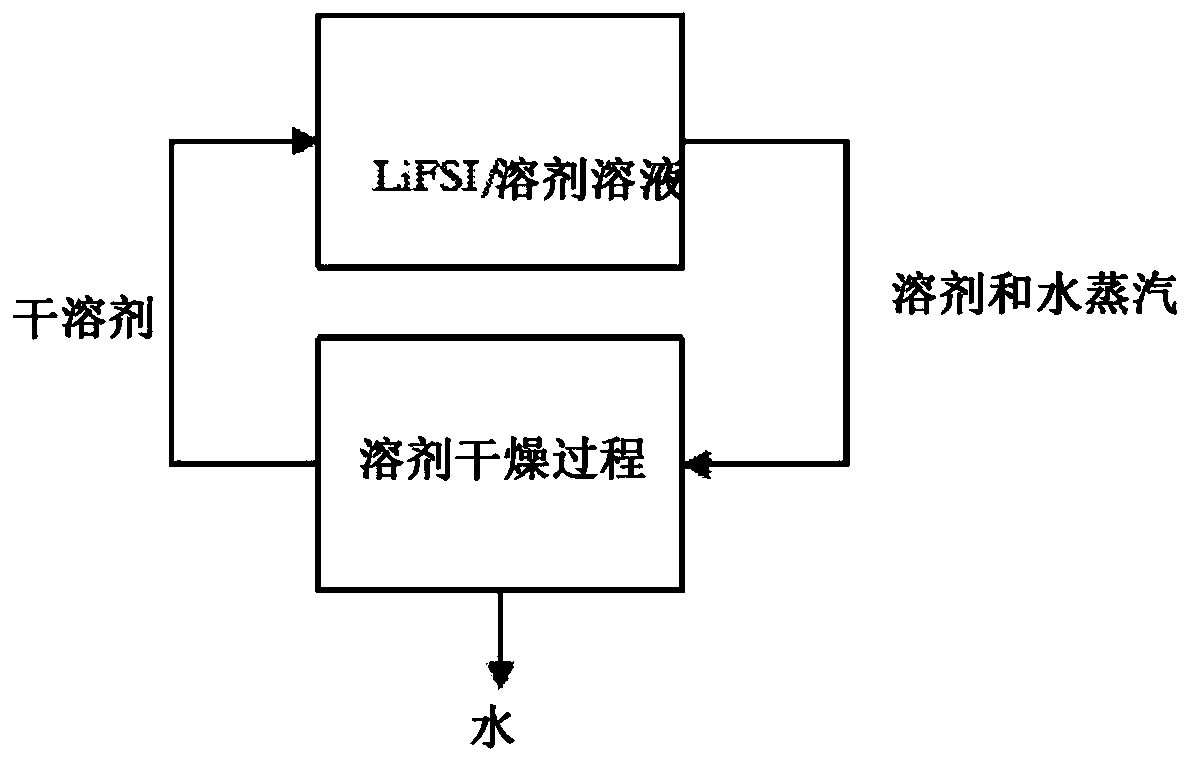
[0085] Example 1: A wet solution of LiFSI and diethyl carbonate (DEC) was prepared by mixing 100 g DEC, 54.6 g LiFSI and 1.59 g water. The solution was found to contain 1.0 wt% water from Karl Fischer titration. This solution was placed in a 1 L three-neck round bottom flask with a magnetic stir bar and heating mantle. One flask neck was closed with a ported stopper through which the thermocouple was immersed in the LiFSI / DEC solution. The other neck was fitted with a 125mL pressure equalizing addition funnel filled with 117.5g of DEC which had 130ppm w Water, as measured by Karl Fischer titration. The final neck is equipped with a vacuum port Liebig distillation head. Connect a 1 L round bottom flask to the receiving end of the distillation head. Immerse the flask in an ice bath. The distillation condenser was cooled to -5°C with an external cooler. A diaphragm vacuum pump was connected to the vacuum port of the distillation head, and the pressure was reduced to about 1...
Embodiment 2
[0087] Example 2: A wet solution of LiFSI and ethyl methyl carbonate (EMC) was prepared by mixing 47.2 g LiFSI, 88.6 g EMC and 1.432 g water. The solution was found to contain 1.06 wt% water by Karl Fischer titration. 123.0 g of this solution was placed in a 300 mL three necked round bottom flask with a magnetic stir bar and heating mantle. The flask was connected to the apparatus described in Example 1. The LiFSI / EMC solution was heated to about 42°C under constant power from a heating mantle. Vacuum was applied at about 30 torr and the condenser was cooled to about -6°C. Add dry EMC to the LiFSI / EMC solution from the addition funnel and observe boiling using condensate collection in the receiving funnel. After adding the contents of the addition funnel, measure the water content of the LiFSI / EMC solution by Karl Fischer titration and refill the addition funnel with dry EMC. Table 1 shows the amount of EMC added and the water concentration in the LiFSI / EMC solution after ...
Embodiment 3
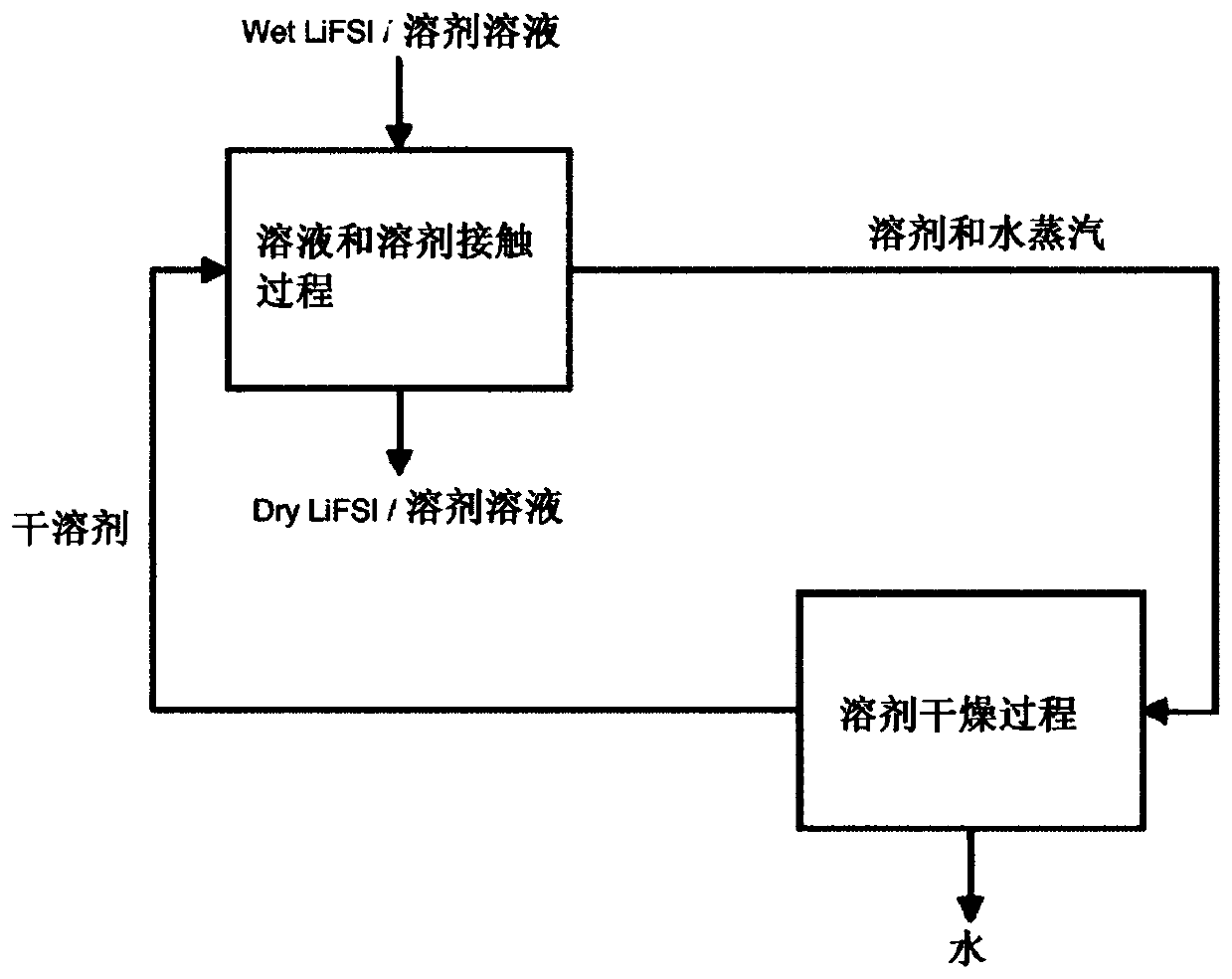
[0092] Example 3 : A solution of LiFSI in water was prepared by mixing HFSI with lithium carbonate. In a jacketed flask equipped with a mechanical stirrer, 814 grams of water and 747 grams of lithium carbonate were added. 3700 g of HFSI was added dropwise to the reactor over the course of 3.5 hours while keeping the reactor temperature below 20°C by circulating a coolant through the jacket. After the addition of HFSI was complete, the reactor contents were drained and filtered to obtain an aqueous solution containing 78 mass % LiFSI. This solution was then mixed with DEC and dried using the method of Example 1.
[0093] Process effluent is CO 2 , water and a small stream of solid impurities (typically <1% of LiFSI production rate). To improve efficiency, the HFSI feed was of high purity, as shown in Table 2. The conversion was almost quantitative as yields above 95% were achieved.
[0094] Table 2. HFSI used in Example 3
[0095] illustrate Value (ppmw) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com