Organic compound, and organic electroluminescent device employing same
An electroluminescent device and organic compound technology, applied in the field of triphenylamine derivatives and organic electroluminescent devices, can solve the problems of low glass transition temperature of blue phosphorescent materials, low material stability, hindering wide use, etc. It is not easy to crystallize and decompose, balance the transfer of holes and electrons, and improve the effect of life.
- Summary
- Abstract
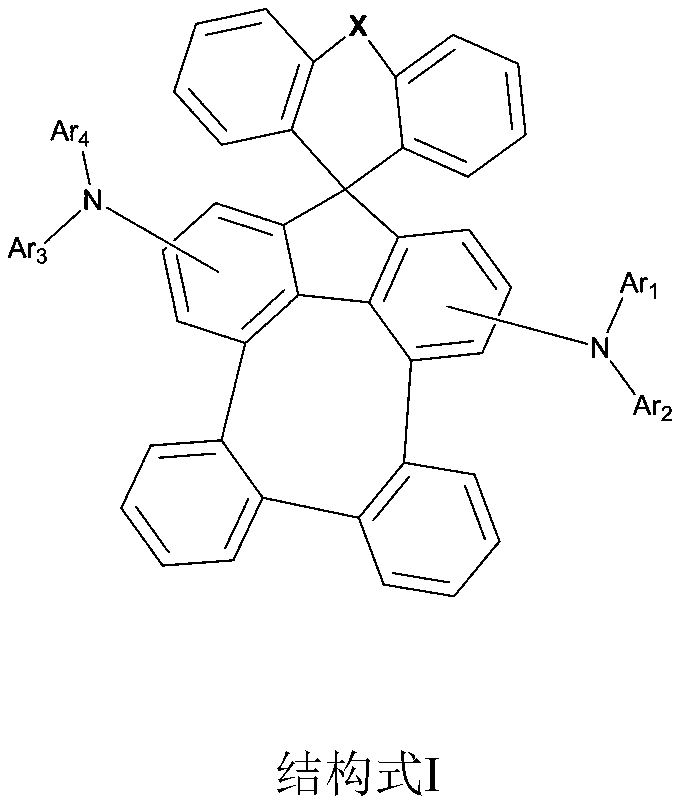
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
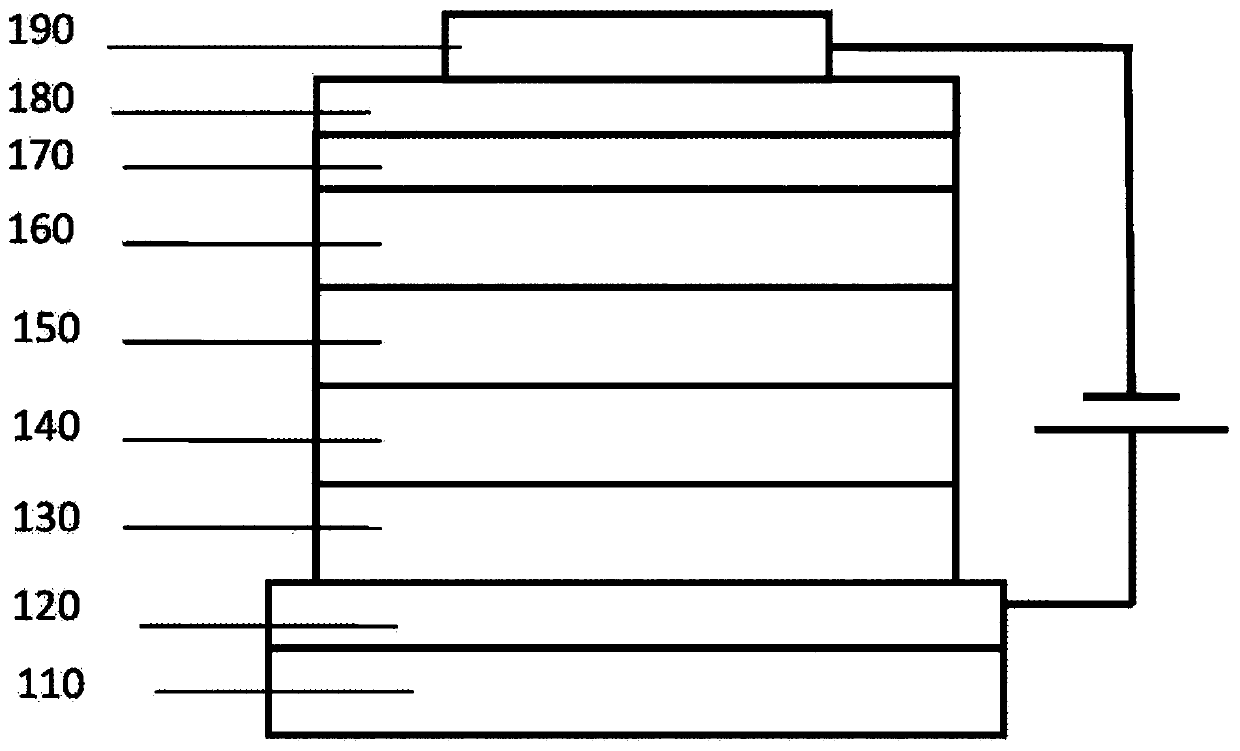
Image
Examples
Embodiment 1
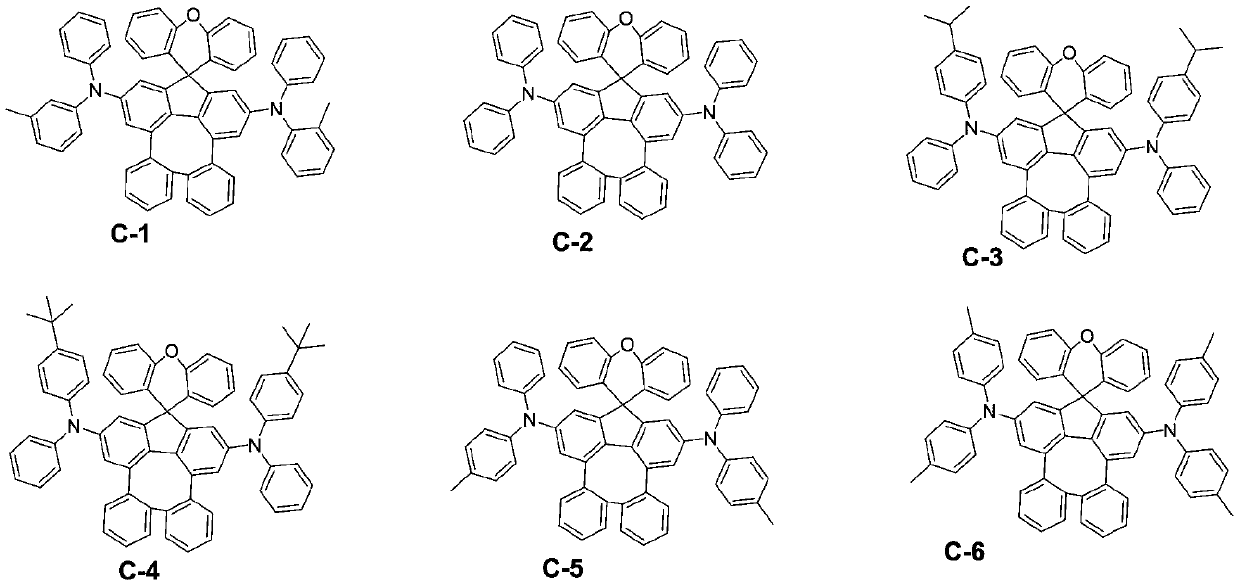
[0055] Synthesis of compound C-6
[0056]
[0057] Add (6.8g, 10.6mmol), bis(4-methylphenyl)amine (5.0g, 25.4mol), Pd 2 (dba) 3 (0.08g, 0.4mmol), sodium tert-butoxide (3.4g, 0.035mol), tri-tert-butylphosphine (0.07g, 0.4mmol), 60mL of toluene, and stirred at reflux for 2 hours. Cool at room temperature after the reaction is complete. The reaction solution was extracted with dichloromethane and water. The organic layer was separated, dried over magnesium sulfate, and concentrated under reduced pressure. The substance was separated and purified by column chromatography, and then recrystallized by dichloromethane and acetone to obtain compound C-6 (3.7 g, 40%). The resulting compound was identified by LC-MS. LC-MS: M / Z 873.3(M+H) + . Theoretical element content (%)C 65 H48N 2O : C, 89.42; H, 5.54; N, 3.21; O, 1.83. The above results confirmed that the obtained product was the target product.
Embodiment 2
[0059] Synthesis of compound C-9
[0060]
[0061] The synthesis steps of compound C-9 are as the above reaction formula, and the preparation and confirmation methods are the same as those of compound C-6, and compound C-9 (4.4 g, 38%) can be obtained. LC-MS: M / Z 1081.4 (M+H)+.
Embodiment 3
[0063] Synthesis of compound C-11
[0064]
[0065] The synthesis steps of compound C-11 are as the above reaction formula, and the preparation and confirmation methods are the same as those of compound C-6, and compound C-9 (4.7 g, 40%) can be obtained. LC-MS: M / Z 1121.4 (M+H)+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com