A kind of serine hydroxymethyltransferase mutant and its application
A serine hydroxymethyl, mutant technology, applied in the field of enzyme catalysis, can solve problems such as high price and limited application, and achieve the effect of improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 Construction of wild-type serine hydroxymethyltransferase expression strain
[0050] 1.1 For the serine hydroxymethyltransferase SHMT (UniProtKB-P0A825) gene derived from Escherichia coli (Escherichia coli), which is SEQ ID NO: 2, entrust Suzhou Jinweizhi Biotechnology Co., Ltd. to synthesize the entire gene sequence of SEQ ID NO: 2, It was constructed into a pSH plasmid (Zhejiang Huarui Biotechnology Co., Ltd.) to obtain an expression vector pSH-SHMT expressing wild-type serine hydroxymethyltransferase SEQ ID NO:1.
[0051] Forward primer SHMT-F: CATATG TTAAAGCGTGAAATGAA
[0052] Reverse primer SHMT-R: GGATCC TTATGCGTAAACCGGGTAAC
[0053] PCR amplifies about 1.2kb fragment, PCR reaction system includes, 0.3μM each primer, 50ng template, 1xKOD Neoplus buffer, 0.2mM dNTP, 1.5mM MgSO 4 , KOD neo plus 1U, add double distilled water to make the total system 50μl.
[0054] PCR conditions: 94°C, 2min; 98°C for 10s, 55°C for 30s, 68°C for 30s, repeated for...
Embodiment 2
[0056] Embodiment 2 constructs SHMT random mutation point library and screening by error-prone PCR method
[0057] 2.1 Construction of SHMT random mutation point library by error-prone PCR method
[0058] Using the sequence SEQ ID NO: 2 as a template, an SHMT random mutant library was constructed using error-prone PCR technology. SHMTmu-F is 5'- ATG TTAAAGCGTGAAATGAA-3', the reverse primer SHMTmu-R is 5'-TTATGCGTAAACCGGGTAAC-3'.
[0059] 50μL error-prone PCR reaction system includes: 50ng plasmid template pSH-SHMT, 30pmol pair of primers SHMTmu-F and SHMTmu-R, 1X Taq buffer, 0.2mM dGTP, 0.2mM dATP, 1mM dCTP, 1mM dTTP, 7mM MgCl 2 , (0mM, 0.05mM, 0.1mM, 0.15mM, 0.2mM) MnCl 2 , 2.5 units of Taq enzyme (Fermentas). The PCR reaction conditions were: 95°C for 5 minutes; 30 cycles of 94°C for 30s, 55°C for 30s, 72°C for 2min / kbp; 72°C for 10min. The 1.2kb random mutation fragment was recovered from the gel as a large primer, and MegaPrimer PCR was performed with KOD-plus DNA po...
Embodiment 3
[0078] Embodiment 3 Mutant bacterial strain construction
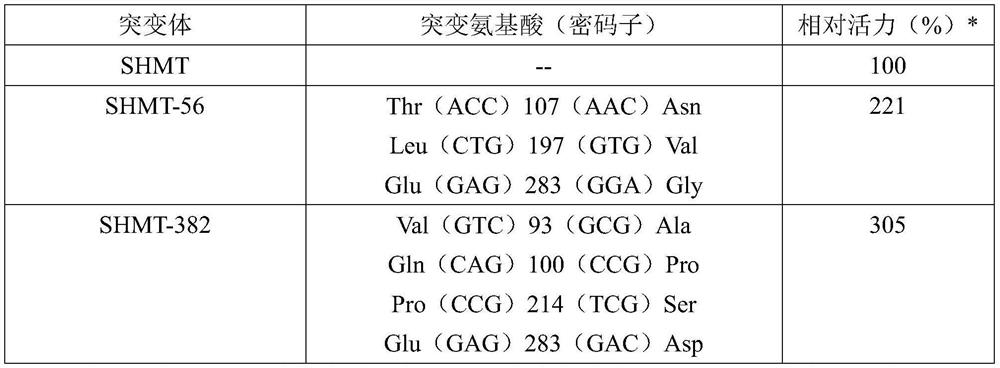
[0079] The focus was on the mutant SHMT-382, and its ability to catalyze the reaction of glycine and formaldehyde to produce L-serine was investigated. To this end, the SHMT-382 gene SEQ ID NO:4 was cloned into the pSH plasmid to obtain the expression vector pSH-SHMT-382 expressing the serine hydroxymethyltransferase mutant SEQ ID NO:3.
[0080] Using the plasmid pSH-SHMT-382, it can be constructed into different expression systems, such as Escherichia coli, Pichia pastoris, and Bacillus subtilis. For example, transform the plasmid pSH-SHMT-382 into BL21(DE3) competent cells, spread on kan+LB plate, culture overnight at 37°C, pick 10 single colonies, inoculate into a test tube containing LB liquid medium, and culture at 37°C Overnight, the bacteria were collected by centrifugation, the plasmid was extracted, and the gene sequence was confirmed to confirm that the mutation was correct, and the engineered bacteria were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com