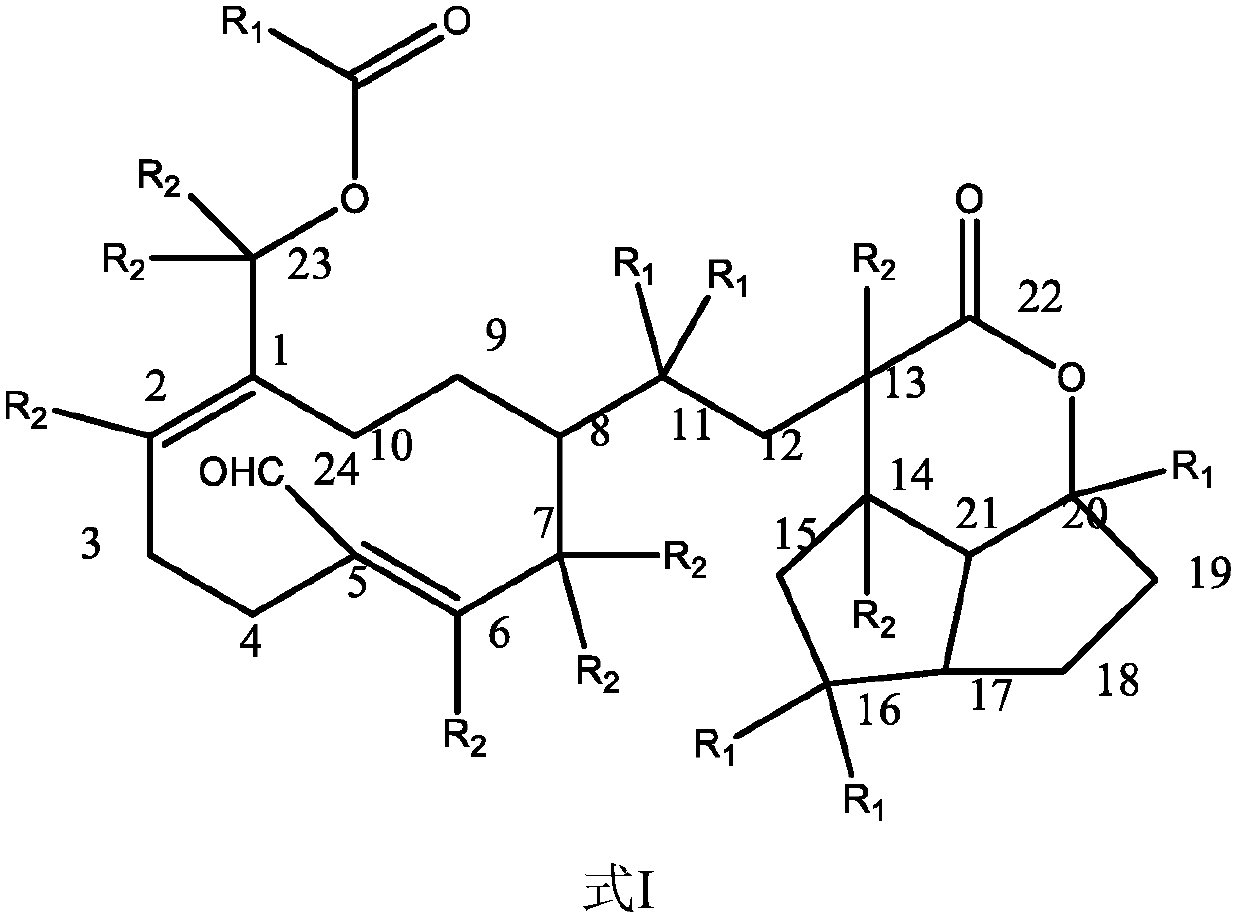
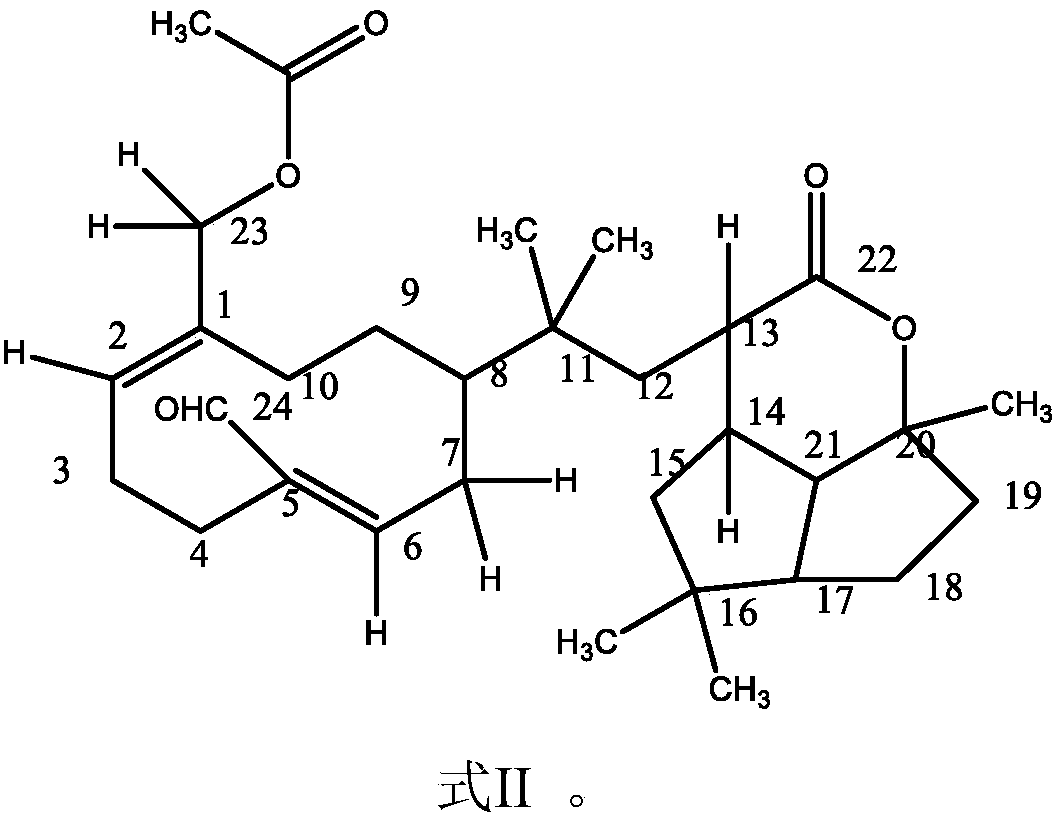
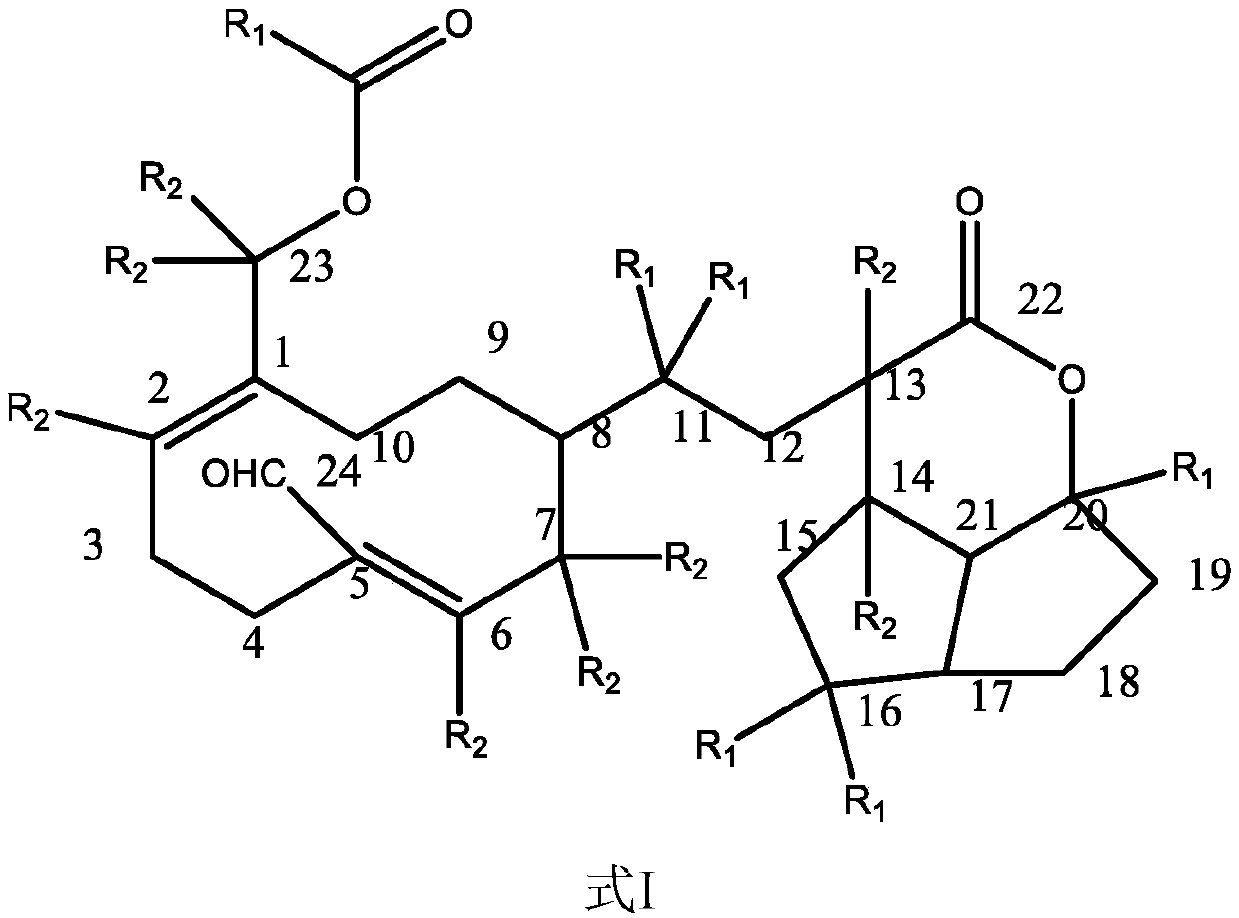
Application of terpene lactone compound for improving gastric motility disorder
A technology of gastric motility disorders and ester compounds, which is applied in the field of pharmaceutical compounds, can solve the problems of large side effects and poor effects, and achieve the effects of improving toxic and side effects, safety of applicable people, and wide application of people
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of compound of formula II
[0052] 1) Extraction: take 20kg of valerian herbal material, pulverize and extract with 50% ethanol (referring to an aqueous ethanol solution with a weight content of 50%) for 2 times of reflux extraction, each time for 2 hours, and the amount of ethanol each time is 120kg.
[0053] 2) Extraction: The ethanol extracts were combined and concentrated under reduced pressure to a clear paste with a relative density of 1.20, then n-hexane was added for extraction, and the n-hexane extract was collected.
[0054] 3) Separation: Weigh 700 g of 200-300 mesh silica gel, dry column packing, use petroleum ether (60-90 ° C) to elute and equilibrate, and pass the above-mentioned extract as a sample solution through a silica gel chromatographic column to make the effective components of the sample solution completely. Adsorbed on silica gel. Then use the mixed solvent of petroleum ether (60-90°C) and ethyl acetate with a volume ratio...
Embodiment 2
[0058] Example 2 Structure identification and analysis of the compound of formula II
[0059] 1) Light yellow oil.
[0060] 2) UV (λ MeOH max ) spectrum, 269nm, indicating the existence of a conjugated system.
[0061] 3) IR(νmax KBr )cm -1 : 3443.84, 2929.24, 2871.71, 1735.32, 1677.59, 1622.83, 1453.35, 1376.10;
[0062] The above data show that there are functional groups such as methyl, methylene, carbonyl, and olefinic bonds in this structure.
[0063] 4) EI-MS (me / z): 291.29, 279.25, 263.31, 234.16, 219.04 (100%), 204.98, 190.95, 178.96, 173.06, 148.92, 144.99, 130.92, 119.11, 107.15, 104.92, 90.9 , 54.94;
[0064] MALDI-TOF MS (me / z): 315.1, 299.1, 273.0, 257.0, 235.0.
[0065] spectrum, 219.04 (100%), but no molecular ion peak is given.
[0066] 5) 1 H-NMR (CDCL 3 , 500MHz) δppm: 9.28(s, 1H), 6.35(d, J=9.5Hz, 1H), 5.35(dd, J=5.5Hz, J=11.5Hz, 2H), 4.18(d, J=12.0Hz, 1H), 4.15 (d, J=12.0Hz, 1H), 2.91 (m, 1H), 2.85 (dd, 1H), 2.26 (m, 1H, C14-H), 2.02 (t, 2H, C12...
Embodiment 3
[0074] Example 3 The effect of the compound of formula II on gastric motility
[0075] The effect of the compound on gastric motility was evaluated by observing the effect of the compound of formula II on gastric emptying in mice loaded with atropine and dopamine.
[0076] (1) The effect of the compound of formula II on gastric emptying of atropine-loaded mice
[0077] SPF grade NIH mice, half male and half male, 18-20g. Mice were randomly divided into 5 groups, namely blank control group, model control group, formula II compound dose group 1 (0.3 mg / kg), formula II compound dose group 2 (0.6 mg / kg), formula II compound dose group 3 ( 1.2 mg / kg). Mice were given intragastric administration (0.2mL / 10g) for 3 days. Before the last administration, mice were fasted for 12 hours, and the test drug was administered by intragastric administration. The blank control group and the model control group were intragastrically administered with an equal volume of water. 20 minutes later,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com