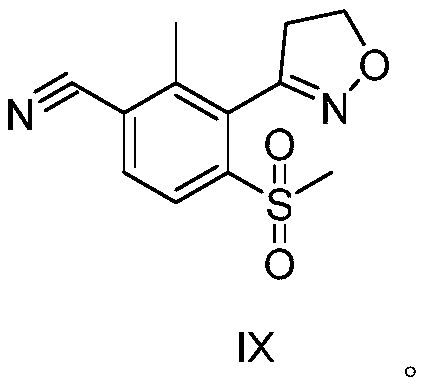
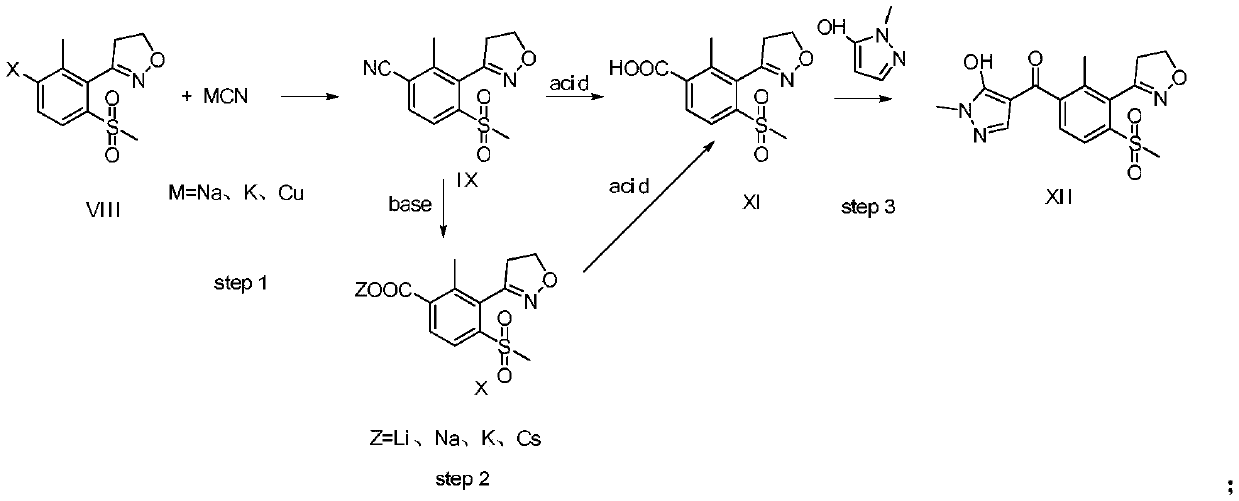
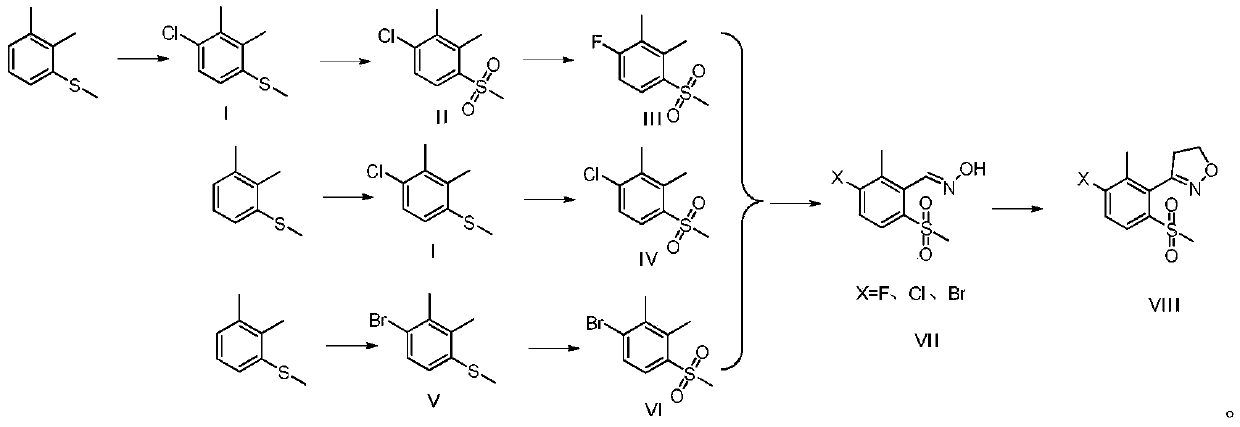
Topramezone intermediate and topramezone preparation method
A technology of fenfentrazone and intermediates, applied in the field of organic synthesis, can solve the problems of increasing the difficulty of industrialization, difficulty in recycling, high price, etc., and achieve the effects of simplifying the process, reducing costs and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] In a 250mL four-necked flask, 160g of chlorobenzene, 40g of 2,3-dimethyl anisole (molecular weight 152.26, 262.71mmol, 1eq), 0.35g of aluminum trichloride (molecular weight 133.34, 2.63mmol, 0.01eq), cool down to 5°C in an ice bath, drop 38.29g of sulfonyl chloride (molecular weight 134.97, 283.73mmol, 1.08eq) within 80 minutes, keep warm in an ice bath for one hour, after the reaction is complete, add 150g of saturated sodium bicarbonate The solution was washed once, then washed with 100g water for the first time, and the organic phase was spin-dried to obtain 48.5g of crude product, which was 2,3-dimethyl-4-methylthiochlorobenzene (molecular weight 186.7, theoretically obtained 49.05g). The rate is 98.88%.
[0052] ( 1 H-NMR (CDCl 3 )δ: 2.35(s,3H), 2.36(s,3H), 2.43(s,3H), 6.97~6.99(d,1H), 7.19~7.21(d,1H)).
[0053]
[0054] Add 48.5g 2,3-dimethyl-4-methylthiochlorobenzene (molecular weight 186.7, 259.78mmol, 1eq) and 1.71g sodium tungstate dihydrate ...
Embodiment 2
[0074] In a 1L four-necked flask, 250g of DCE and 83.33g of 2,3-dimethylanisole (molecular weight: 152.26, 0.574mol, 1eq) were sequentially added at room temperature, and stirred to dissolve. Maintain 20-25°C and start to add 87.07g of bromine (molecular weight 159.08, 0.574mol, 1eq) dropwise, and drop it for about 1 hour. After the dropwise addition was completed, the mixture was incubated and stirred at room temperature for one hour. The reaction solution was washed once with 60g water and 30g water successively, the organic phase was separated, and the solvent was evaporated to dryness under negative pressure to obtain 124.51g of crude product, which was 2,3-dimethyl-4-methylthiobromobenzene (molecular weight 231.15, theoretically obtained 126.51g), mass yield 98.42%.
[0075] ( 1 H-NMR (CDCl 3 )δ: 2.36(s,3H), 2.40(s,3H), 2.42(s,3H), 6.89~6.91(d,1H), 7.36~7.38(d,1H)).
[0076]
[0077] Add 249.02g acetic acid, 124.51g 2,3-dimethyl-4-methylthiobromobenzene (molecular ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com