Paradol synthesis method
A synthesis method and a technology for zingerol, which are applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, preparation of carbon-based compounds, etc., can solve the problem that compound 21 has a long reaction time, high cost, and is not suitable for industrialization. Production and other problems, to avoid absolute anaerobic conditions, low cost, stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
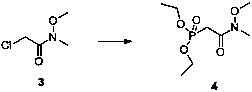
[0093] Preparation of compound 3
[0094]
[0095] Add methoxymethylamine hydrochloride (20.0 g, 211 mmol, 1.0 eq.), dichloromethane (100 mL), chloroacetyl chloride aqueous solution (ca. 2.1 M H2O solution, 100 mL, 211 mmol, 1.0eq.), cooled to 0ºС in an ice bath, and added anhydrous potassium carbonate (138.21 g, 253 mmol, 1.2 eq.) in portions. After the addition was completed, the reaction was continued at room temperature for 12 h, detected by TLC. After the reaction was complete, the reaction solution was poured into 200 mL of water, and the aqueous phase was extracted with dichloromethane (50 mL×3). The organic phases were combined, dried over anhydrous sodium sulfate, the filtrate was collected by filtration, and spin-dried to obtain 32.3 g of a colorless transparent oil, with a yield of 93%. 1H NMR (400 MHz, CDCl3) δ 4.25 (s, 2H, CH2), 3.76 (s, 3H, NCH3), 3.24 (s, 3H, OCH3); 13C NMR (100 MHz, CDCl3) δ 167.62 (C=O ), 61.77,40.81, 32.58; HRMS (ESI): calcd. for C4H8ClN...
Embodiment 2
[0112] Preparation of compound 3
[0113]
[0114] Add methoxymethylamine hydrochloride (1.0 kg, 10.2 mol, 1.0 eq.), dichloromethane (4 L), chloroacetyl chloride aqueous solution (ca. 3 M H2O solution, 3.4 L, 10.2 mol, 1.0 eq.), cooled to -5 ℃ under ice bath, and added anhydrous potassium carbonate (1.7 kg, 12.2 mol, 1.2 eq.) in batches. After the addition was completed, the reaction was continued at room temperature for 12 h, and detected by TLC. After the reaction was complete, the reaction solution was poured into water (5 L), and the aqueous phase was extracted with dichloromethane (2 L×3). The organic phases were combined, dried over anhydrous sodium sulfate, the filtrate was collected by filtration, and spin-dried to obtain 1.3 kg of a colorless transparent oil, with a yield of 94%. HRMS (ESI): calcd. for C4H8ClNO2 [M+H]+, 138.0244; found 138.0278.
[0115] Preparation of Compound 4
[0116]
[0117] Add compound 3 (1.1 kg, 8.0 mol, 1.0 eq.) and triethyl phosphi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com