Coding gene of duck costimulatory molecule CD40 and application of coding gene
A technology of co-stimulatory molecules and coding genes, which is applied in the fields of application, genetic engineering, plant gene improvement, etc., to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
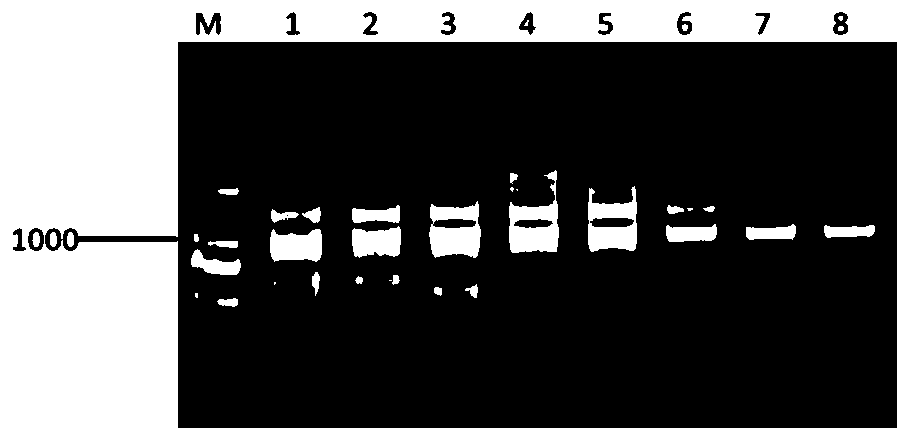
[0031] Example 1 Cloning of the full-length sequence of duck co-stimulatory molecule CD40
[0032] 1.1 Cloning of the 3' end CDS of duck CD40
[0033] 1.1.1 Primer design
[0034] According to the incomplete predicted sequence of duck CD40 found in GenBank (GenBank ID: XM_021276409.1) and the predicted sequence of goose CD40 (GenBank ID: XM_013198462.1) with high homology to the predicted sequence of duck CD40, a pair of primers were designed, upstream Primer: 5'-CCAAAACCGAGCCGTGCTACCCC-3'; downstream primer: 5'-C GCCCTGCGCCCCTCACAGC-3'. After the primers were synthesized, they were dissolved in an appropriate amount of sterilized deionized water to make the final concentration 10 μM, and stored at -20°C for later use.
[0035] 1.1.2 Extraction of duck blood RNA
[0036] (1) Take an appropriate amount of duck blood and add it to a 1.5mLEP tube, add 1mL of Trizol Reagent, shake vigorously until it is completely lysed, and then let it stand at room temperature (25-27°C) for 1...
Embodiment 2
[0076]Preparation of embodiment 2 duck CD40 recombinant protein
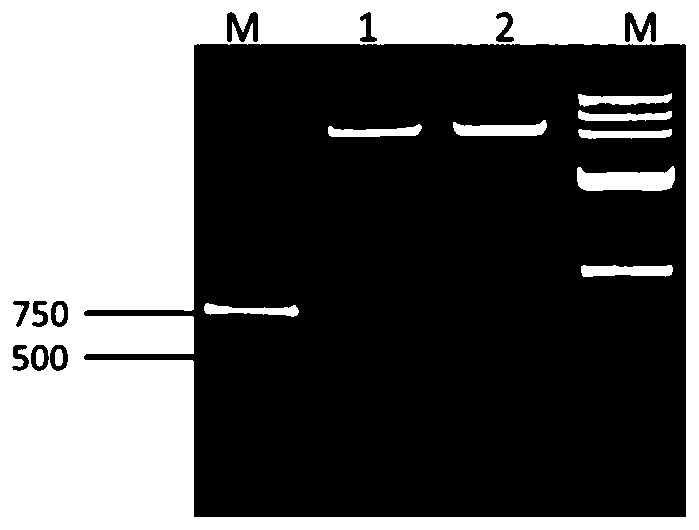
[0077] 2.1 Construction of prokaryotic expression plasmid pET28a-CD40, induction of expression and optimization of expression conditions
[0078] 2.1.1 Primer design
[0079] According to the nucleotide sequence corresponding to the protein sequence encoded by duck CD40, a pair of primers were designed, the upstream primer: 5'-CGGAATTCATGCACTGCTCAGACACCAGC-3'; the downstream primer: 5'-CCGCTCGAGTCACAGCCCCTCCTGCTCC-3'. After the primers were synthesized, they were dissolved in an appropriate amount of sterilized deionized water to make the final concentration 10 μM, and stored at -20°C for later use.
[0080] 2.1.2 Construction and identification of prokaryotic expression plasmid pET28a-CD40
[0081] Using pMD19T-CD40 as a template, amplify the target fragment with the primers described in 2.1.1, and recover and purify it. Then the target fragment and pET28a(+) empty plasmid were subjected to double enzyme dig...
Embodiment 3
[0116] Example 3 Preparation and application of duck CD40 recombinant protein polyclonal antibody
[0117] 3.1 Preparation of polyclonal antibody against duck CD40 recombinant protein
[0118] 3.1.1 Animal immunization procedures
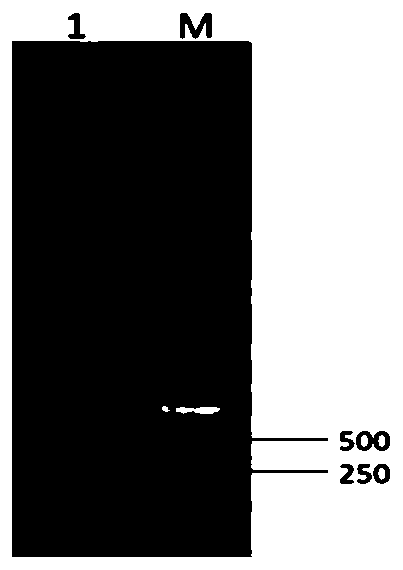
[0119] Using the purified duck CD40 recombinant protein as the antigen, mix it with an equal amount of Freund's complete adjuvant, shake vigorously to fully emulsify the antigen into a water-in-oil emulsion seedling. Twelve healthy female Kunming rats were taken, and each rat was intradermally injected with the aforementioned emulsion vaccine (50 μg of duck CD40 recombinant protein) for primary immunization. Two weeks after one immunization, the purified duck CD40 recombinant protein was mixed with an equal amount of Freund's incomplete adjuvant, vigorously shaken to emulsify the antigen fully into a water-in-oil emulsion vaccine, and then each mouse was subcutaneously injected with the emulsion vaccine (duck The amount of CD40 recombinant protein...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com