Photo-bleachable visible light initiator as well as preparation method and application thereof
A technology of visible light and initiator, which is applied in the field of photobleachable visible light initiator and its preparation, can solve the problems of reduced curing depth of curing system and reduced visible light penetration, and achieves simple synthesis method, wide applicability and matching reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
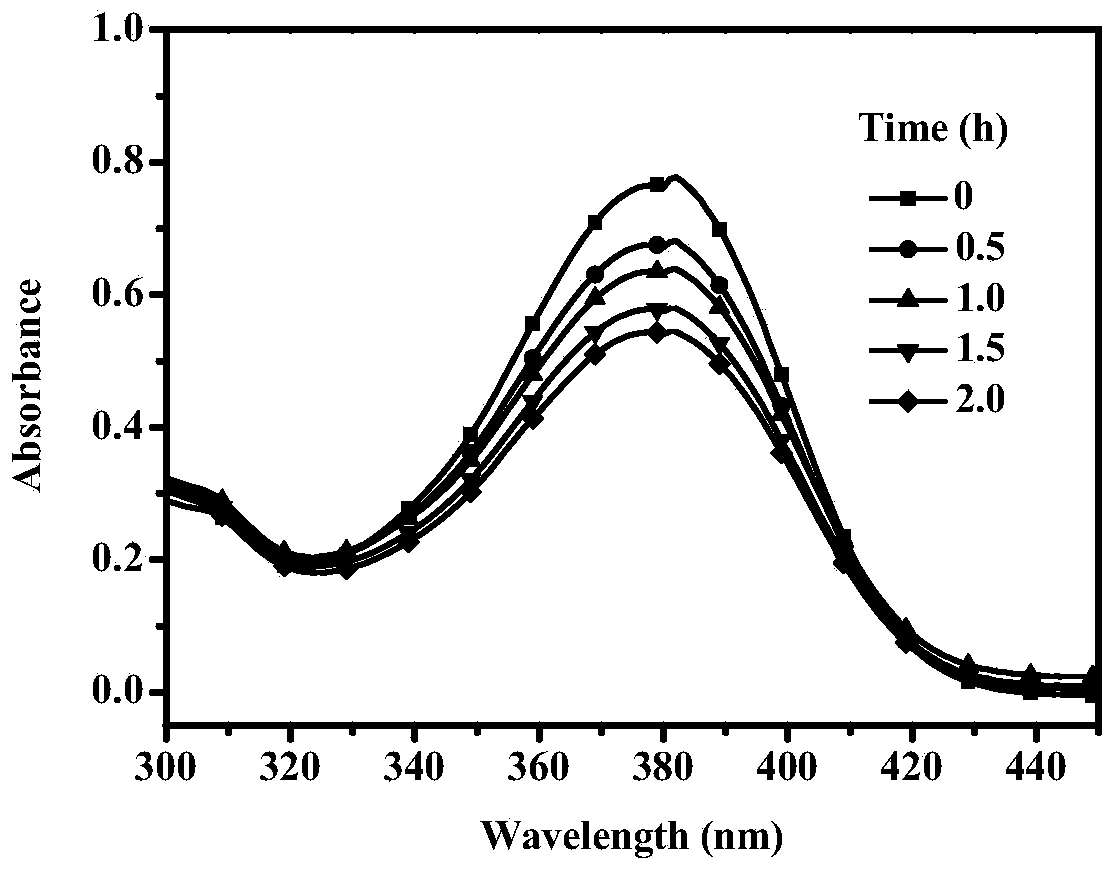
Method used
Image
Examples
Embodiment 2
[0046] [Example 2] Preparation of photobleachable visible light initiator shown in formula 2
[0047]
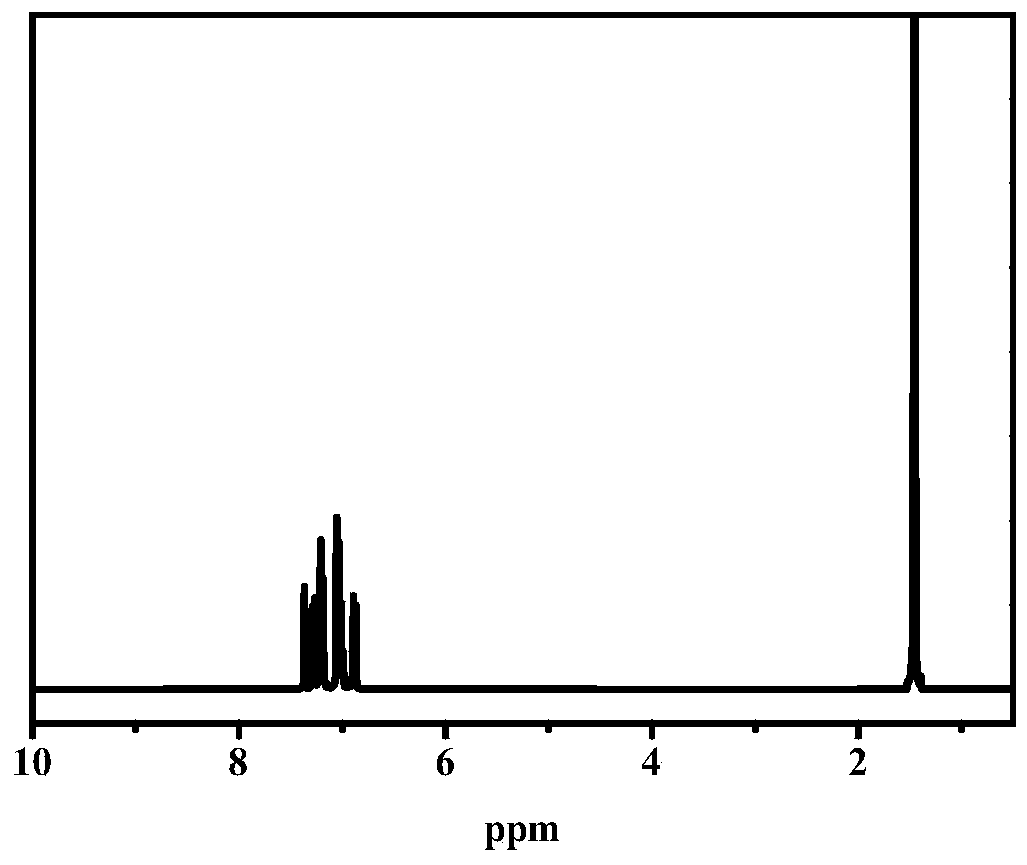
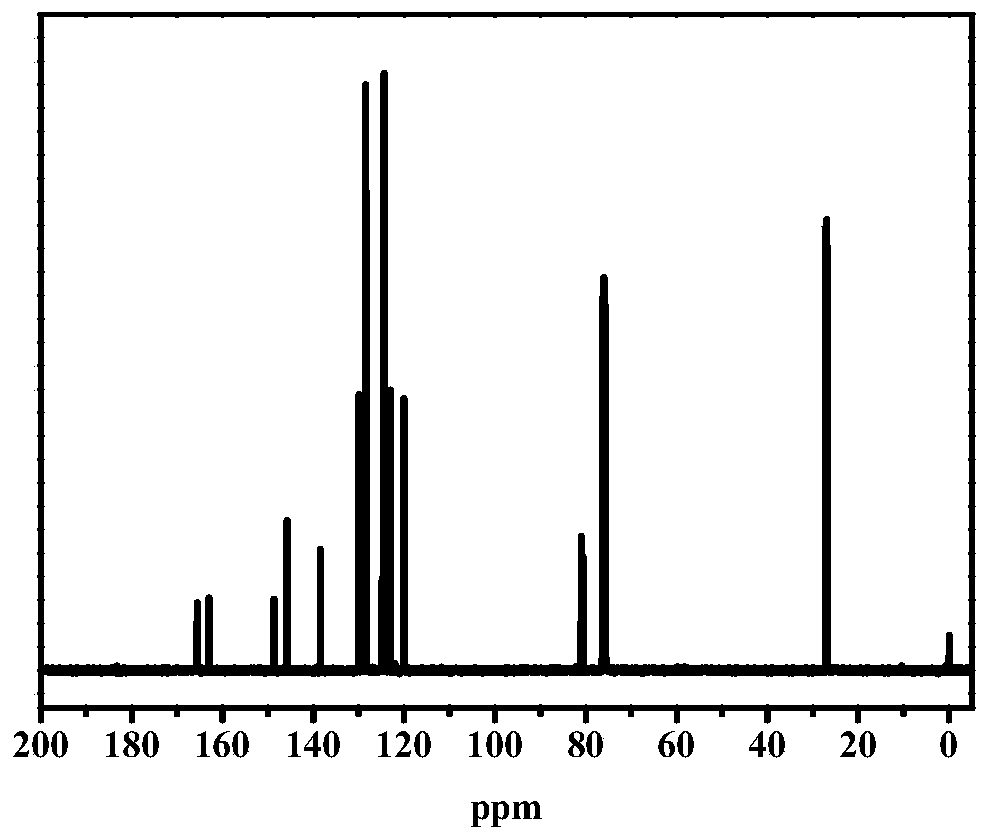
[0048] Add 2-allyl-4-diethylaminobenzaldehyde (0.217g, 1mmol) in 100mL single-necked flask, cyclohexylamine (0.1g, 1mmol), diethyl malonate (0.216g, 1mmol), Glacial acetic acid (0.06g, 1mmol), 10g of toluene, heated up to 100°C for 48h under the protection of argon, the raw materials were almost completely reacted by TCL, the low boiling point was removed under reduced pressure, diluted by adding PE:EA=10:1, centrifuged, The supernatant was passed through a silica gel column (PE:EA=10:1) to obtain a yellow transparent liquid, which was a photobleachable visible photoinitiator. 1 H NMR (400MHz, CDCl 3 )δ8.62(s,1H,-C=CH-Ar),7.28(d,J=8.7Hz,1H,Ar-H),7.20(dd,J=14.2,6.6Hz,2H,Ar-H) ,5.92(dd,J=16.8,7.8Hz,1H,-CH 2 CH=CH 2 ),5.04(d,J=8.7Hz,2H,-CH 2 CH=CH 2 ),4.29–4.18(m,4H,-OCH 2 CH 3 ),3.36(q,J=7.1Hz,4H,-NCH 2 CH 3 ),3.14–2.96(m,2H,-CH 2 CH=CH 2 ), 1.23 (dt, J=11.7, 7....
Embodiment 3
[0049] [embodiment 3] the preparation of photobleachable visible light initiator shown in formula 3
[0050]
[0051] Sodium hydride (0.072g, 3mmol) was added to a 100mL single-necked flask, placed in an ice-water bath, and 2,4-diethylaminobenzaldehyde (0.248g, 1mmol) was slowly added dropwise under the protection of argon, anhydrous ethyl acetate Ester (0.55g, 3mmol), mixed solution of anhydrous tetrahydrofuran 5.0g, dropwise addition was completed in 30min, reacted in an ice-water bath for 12h, TCL detected that the raw material had reacted completely, removed the low boiling point under reduced pressure, added PE:EA=10:1 to dilute, after Silica gel column (PE:EA=10:1) gave yellow transparent oily liquid. 1 H NMR (400MHz, CDCl 3 )δ8.08(d,J=16.1Hz,1H,-C=CH-Ar),7.65–7.36(m,1H,Ar-H),6.43–6.33(m,1H,Ar-H),6.30( d,J=2.6Hz,1H,HC=C-),6.19(d,J=16.0Hz,1H,Ar-H),4.35–4.14(m,2H-OCH 2 CH 3 ),3.37(q,J=7.1Hz,4H,-NCH 2 CH 3 ), 3.04 (q, J=7.1Hz, 4H, NCH 2 CH 3 ), 1.31 (q, J=7.2Hz, ...
Embodiment 6
[0058] [embodiment 6] the preparation of photobleachable visible light initiator shown in formula 6
[0059]
[0060] Add 2,4-bis-(diethylamino)benzaldehyde (0.248g, 1mmol) in 100mL single-necked flask, cyclohexylamine (0.1g, 1mmol), 1,3-indenedione (0.146g, 1mmol), Glacial acetic acid (0.06g, 1mmol), 10g of toluene, under the protection of argon at room temperature for 12h, the raw materials were almost completely reacted by TCL, the low boiling was removed under reduced pressure, diluted by adding PE:EA=10:1, centrifuged, and the supernatant was passed through Silica gel column (PE:EA=10:1) to obtain a red solid powder is photobleachable visible light initiator. 1 H NMR (400MHz, CDCl 3 )δ9.12–8.87(m,1H,-C=CH-),8.18(s,1H,Ar-H),7.96–7.69(m,2H,Ar-H),7.69–7.42(m,2H, Ar-H), 6.34(dt, J=22.3, 11.2Hz, 1H, Ar-H), 6.11(dd, J=12.0, 2.5Hz, 1H, Ar-H), 3.47–3.26(m, 4H,- NCH 2CH 3 ), 3.11 (q, J=7.1Hz, 4H, -NCH 2 CH 3 ),1.22–1.12(m,6H,-NCH 2 CH 3 ), 1.07(t, J=7.1Hz, 6H, -NCH 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com