Ruthenium-copper bimetallic catalyst, preparation method and application thereof
A bimetallic catalyst and aniline technology are applied in the synthesis field of ruthenium-copper bimetallic catalysts, can solve the problems of multiple solid wastes, high reaction cost, complicated steps, etc., and achieve the effects of wide application prospect, high catalyst activity, and reaction condition temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Catalyst preparation:
[0028] Configure 100mL of mixed aqueous solution of ruthenium chloride and copper chloride, wherein the concentration of ruthenium is 0.4mol / L, and the concentration of copper is 0.6mol / L; 1:9), hydrochloric acid mixed aqueous solution 100mL, in which the concentration of substituted aniline is equal to the concentration of hydrochloric acid, both are 1.5mol / L. Mix an equal volume (100 mL) of ruthenium-copper mixed aqueous solution with hydrochloric acid solution of substituted aniline, adjust the pH value to neutral with milk of lime (ie, calcium hydroxide), and then blow in air at a rate of 0.8 mL / s for 24 hours to obtain The ruthenium-copper bimetallic catalyst was obtained after drying the precipitate at 90°C. The weight ratio of the obtained catalyst weight to the substituted aniline used is 0.98 (various impurities are mixed in the reaction, it is difficult to calculate the exact yield, so the polymer / monomer weight ratio is used to descri...
Embodiment 2
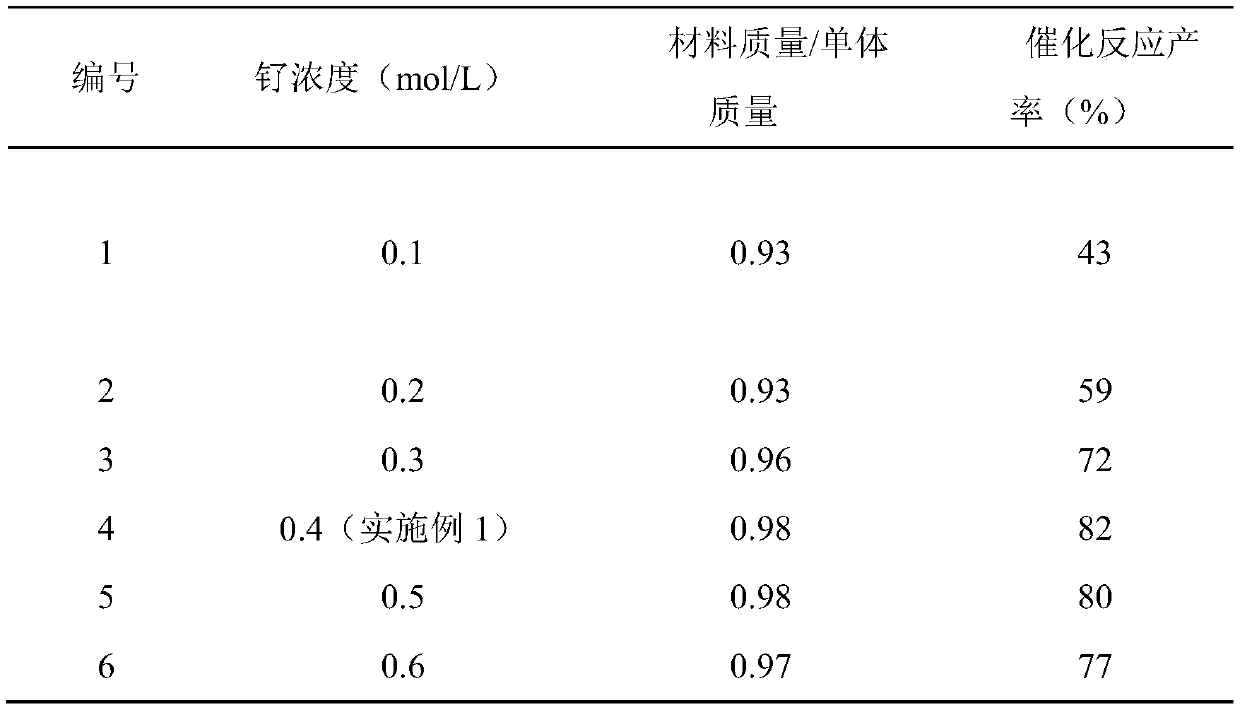
[0032] Other conditions are the same as in Example 1, and the properties of materials synthesized by using different concentrations of ruthenium chloride are tested, and the experimental results are shown in Table 1.
[0033] The performance test of the material synthesized by different ruthenium concentrations in table 1
[0034]
[0035] From the above results, it can be seen that the catalytic effect of the material synthesized using 0.4mol / L ruthenium chloride (Example 1) is the best (No. 4), and the concentration of ruthenium chloride has little influence on the output of the catalytic material.
Embodiment 3
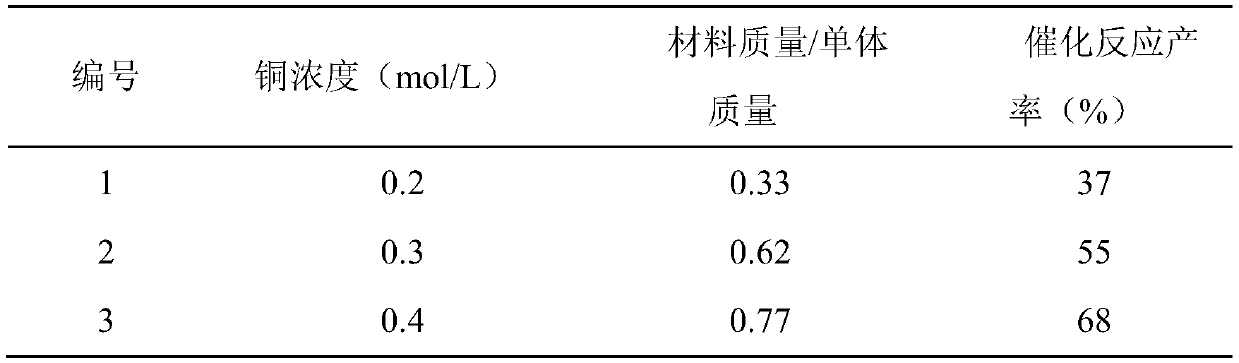
[0037] Other conditions are the same as in Example 1, and the effect of different copper chloride concentrations is checked, and the experimental results are as shown in Table 2.
[0038] The performance test of the material synthesized by different copper concentrations in table 2
[0039]
[0040]
[0041] As can be seen from the above results, the catalytic effect of the material synthesized using 0.6mol / L cupric chloride (Example 1) is the best (No. 5), and the concentration of copper increases, although the output of the catalyst material will increase, but the activity drops sharply.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com