Magnesium single-carrier in-situ supported non-metallocene catalyst and preparation method and application thereof
A non-metallocene and non-metallocene ligand technology, applied in the field of non-metallocene catalysts, can solve the problems of affecting the final catalyst activity, restricting the use of polymers, and low olefin polymerization activity, so as to achieve the improvement of catalytic activity and polymer bulk density , significant copolymerization effect and low amount of co-catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
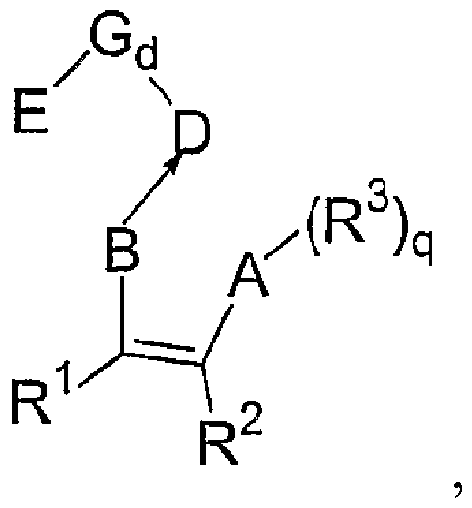
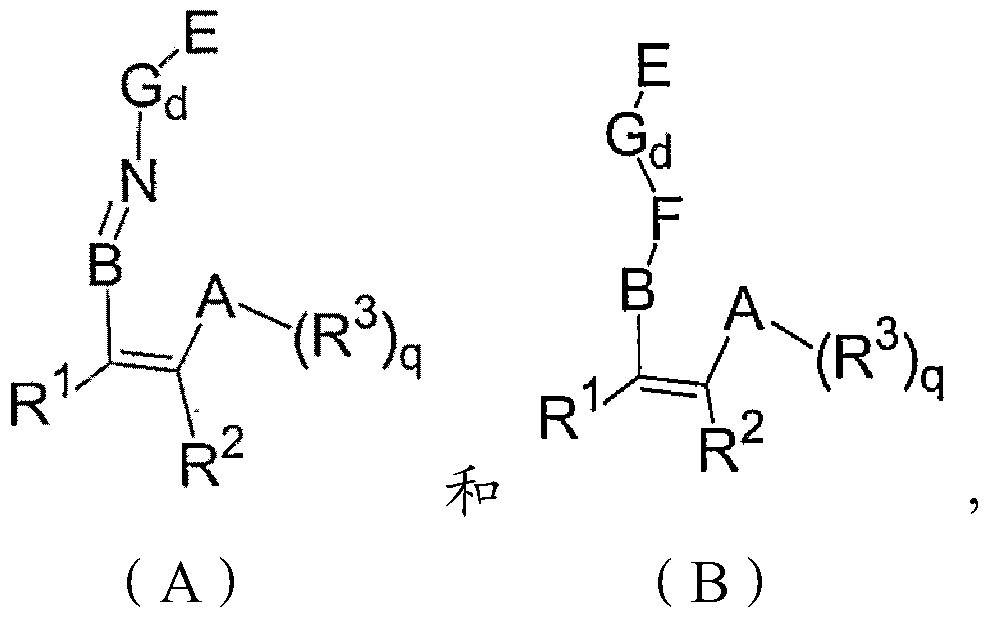
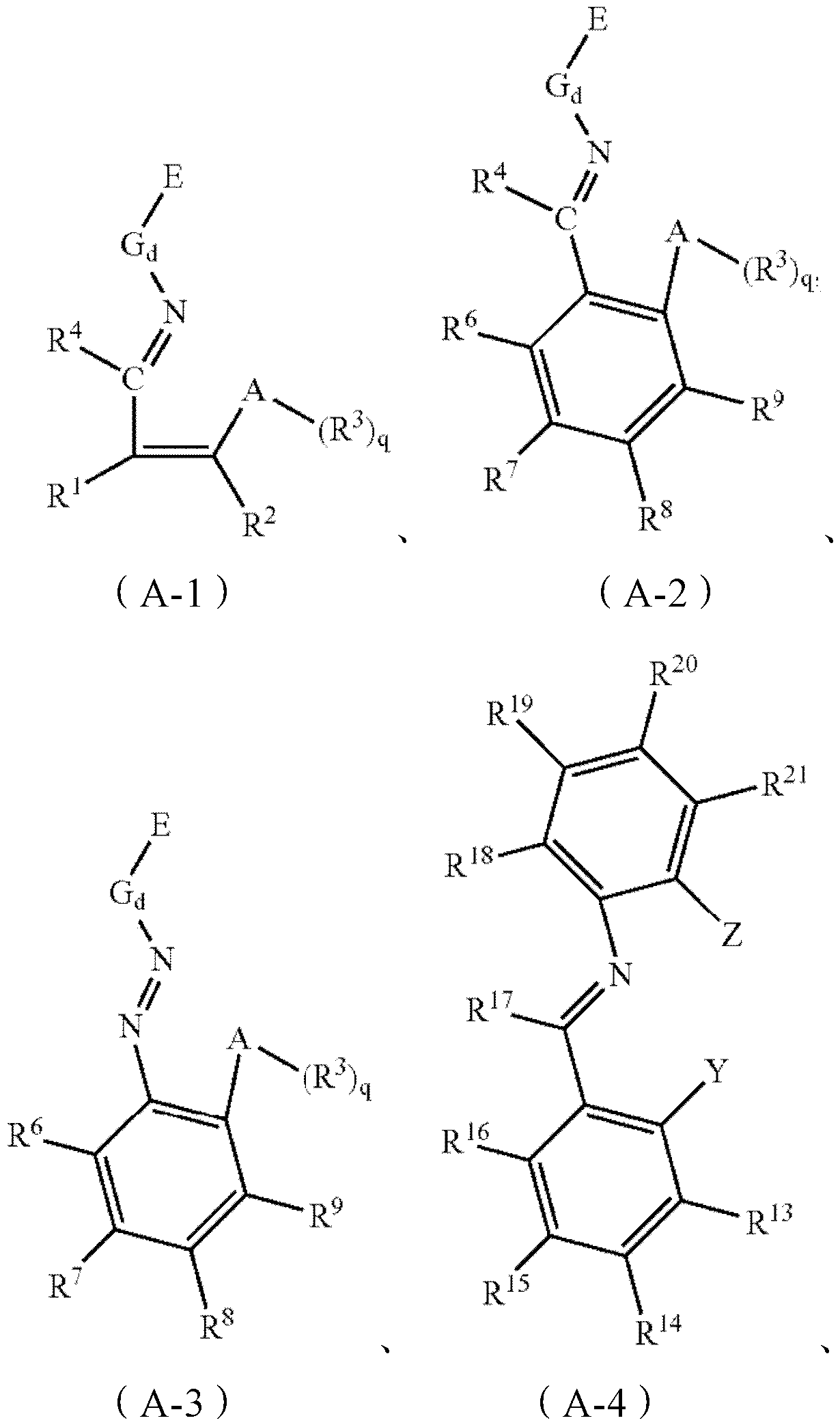
[0030] The invention relates to a preparation method of a magnesium single carrier in-situ supported non-metallocene catalyst, comprising the following steps:
[0031] Dissolving the magnesium compound and the non-metallocene ligand in tetrahydrofuran to obtain a magnesium compound solution; drying the magnesium compound solution, or adding a precipitating agent to the magnesium compound solution and drying the resulting solid product to obtain a modified carrier step, wherein the tetrahydrofuran content in the modified carrier is 0.10-0.50wt%; and the modified carrier is treated with a chemical treatment agent selected from group IV B metal compounds to obtain the magnesium single carrier in situ loaded amphocene Metal catalyst steps.
[0032] According to the invention, no alcohol is used in said step of obtaining the magnesium compound solution.
[0033] The steps for obtaining the magnesium compound solution are specifically described below.
[0034] According to this proc...
Embodiment 1
[0227] Weigh 2.5g magnesium compound anhydrous magnesium chloride (MgCl 2 ), add a certain amount of tetrahydrofuran, heat to 60 ° C to dissolve, add a certain amount of non-metallocene ligands, continue stirring at 60 ° C to dissolve completely, and obtain a magnesium compound solution. After stirring for 2 hours, add a precipitant hexane to make it precipitate , filtered, and the solid product was collected, washed twice with hexane, and the amount of hexane added each time was the same as that added before, and the obtained solid product was dried at 45 °C and an absolute pressure of 10 mBar for 6 h, and then at 80 °C and Drying under a vacuum of 10 mBar for 8 hours obtained a modified carrier, wherein the tetrahydrofuran content was 0.17 wt%.
[0228] Measure 25ml of hexane solvent, join in the modified carrier, add titanium tetrachloride (TiCl) dropwise in 15 minutes under stirring condition 4 ) the hexane solution of the chemical treatment agent, after reacting at 30°C ...
Embodiment 2
[0233] Basically the same as Example 1, but with the following changes:
[0234] Without using a precipitating agent, the magnesium compound solution was dried at 35° C. and an absolute pressure of 10 mBar under vacuum for 10 h, and then at 90° C. and an absolute pressure of 10 mBar under vacuum for 6 h to obtain a modified carrier, wherein the tetrahydrofuran content was 0.13 wt%.
[0235] This catalyst is designated CAT-2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com