Quality control product of inflammation marker and preparation method
A technology of quality control products and markers, applied in the field of clinical medical testing, to achieve the effects of easy promotion and use, high compliance, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of inflammation quality control product described in the present invention, comprises the steps:
[0035] (1) Treatment of quality control matrix solution: reconstitute commercial human serum or plasma and return to room temperature, then place in a water bath at 42-65°C for 1-3 hours.
[0036] (2) Preparation of quality control matrix solution: add HEPES buffer 5-100mmol / L to adjust the pH to 5.0-9.0, add 0.01-5g / L aminopyrine, 0.05-5g / L Proclin300, 5-100g / L L trehalose, mixed evenly;
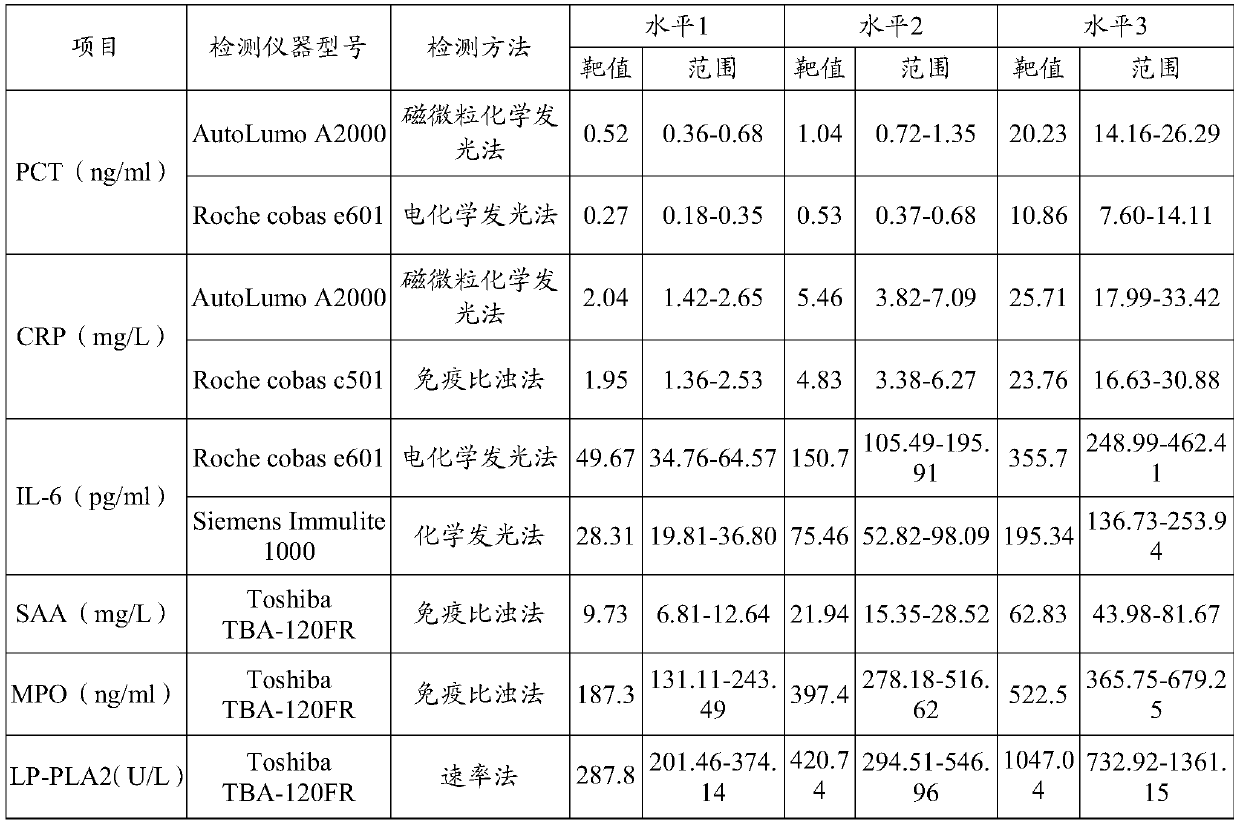
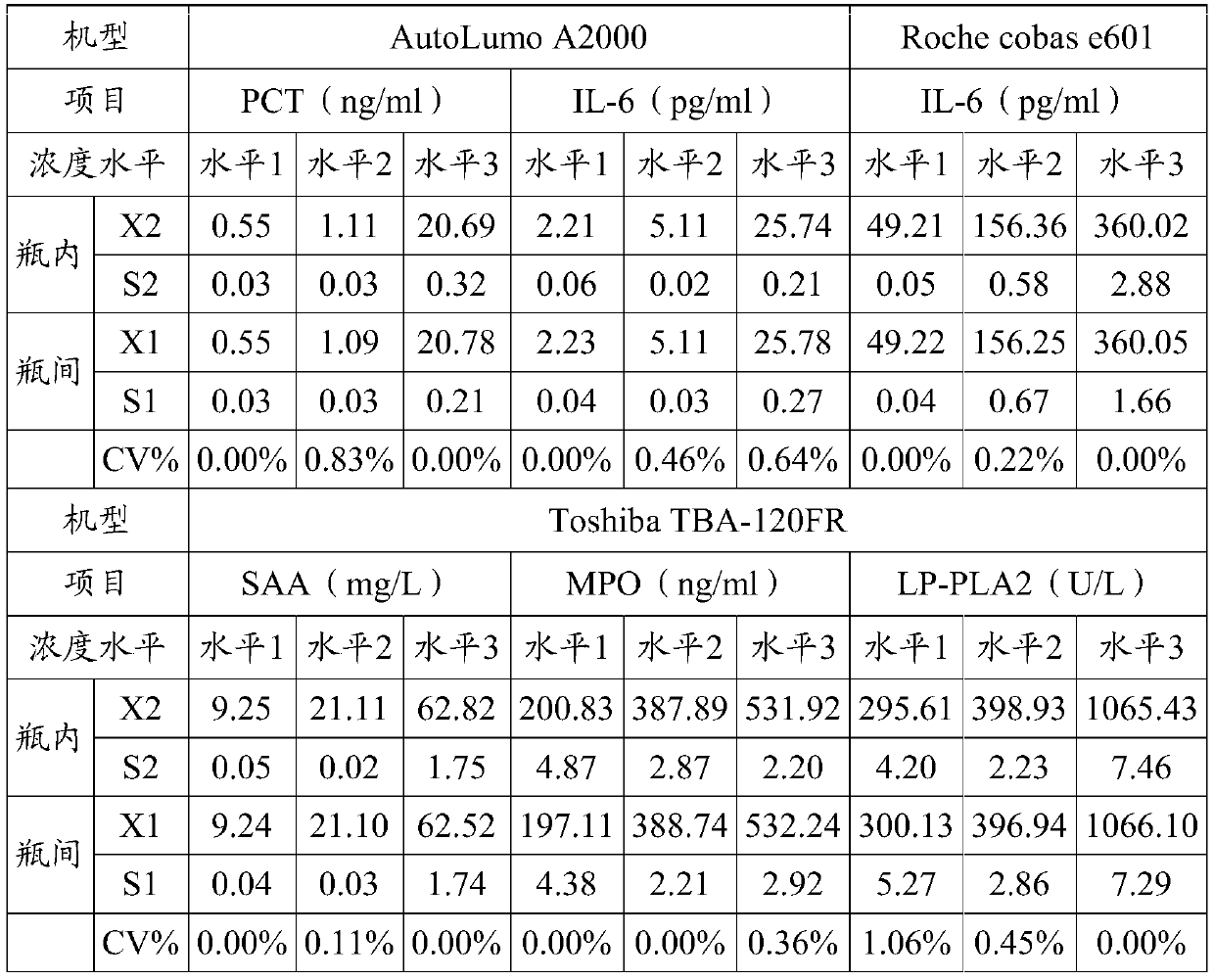
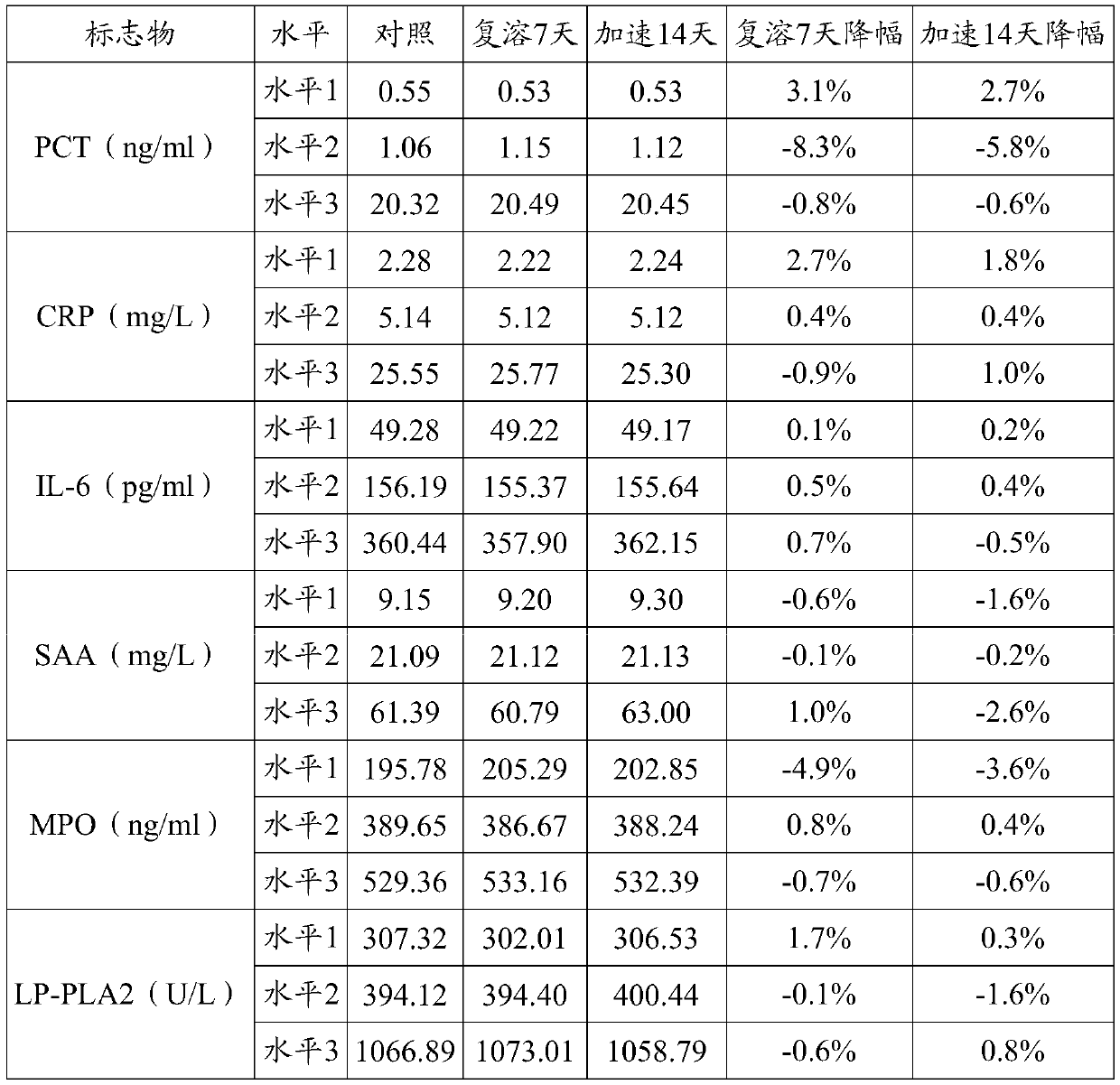
[0037]3) Preparation of quality control products: divide the prepared quality control product matrix solution into 3 levels of high, middle and low, and add positive high values of inflammatory markers according to the content of the following inflammatory markers for testing: Procalcitonin (PCT) 0.01 -100ng / ml, C-reactive protein (CRP) 0-300mg / L, serum amyloid A (SAA) 10-200mg / L, myeloperoxidase (MPO) 50-1000ng / ml, interleukin-6 (IL -6) 10-1000 pg / ml, lipopro...
Embodiment 1
[0045] Embodiment 1: Inflammation marker substance control product of the present invention
[0046] The matrix solution is composed of the following components: normal human serum, 50mmol / L HEPES buffer, 2.0g / L aminopyrine, 50g / L trehalose, 2.0g / L Proclin300;
[0047] The content of the inflammatory markers:
[0048] 1) The content of the inflammatory markers in the high-level inflammation composite quality control product Procalcitonin (PCT) 20ng / ml, C-reactive protein (CRP) 25mg / L, serum amyloid A (SAA) 50mg / ml L. Myeloperoxidase (MPO) 500ng / ml, interleukin-6 (IL-6) 350pg / ml, lipoprotein-associated phospholipase (LP-PLA2) 1000μg / L;
[0049] 2) The contents of the inflammatory markers in the medium-level inflammation composite quality control product are procalcitonin (PCT) 1ng / ml, C-reactive protein (CRP) 5mg / L, serum amyloid A (SAA) 20mg / ml L. Myeloperoxidase (MPO) 400ng / ml, interleukin-6 (IL-6) 150pg / ml, lipoprotein-associated phospholipase (LP-PLA2) 400μg / L;
[0050] ...
Embodiment 2
[0051] Embodiment 2: Inflammation marker substance control product of the present invention
[0052] The matrix solution is composed of the following components: normal human plasma, 10mmol / L HEPES buffer, 1.0g / L aminopyrine, 30g / L trehalose, 1.0g / L Proclin300;
[0053] The content of the inflammatory markers:
[0054] 1) The content of the inflammatory markers in the high-level inflammation composite quality control product Procalcitonin (PCT) 20ng / ml, C-reactive protein (CRP) 25mg / L, serum amyloid A (SAA) 50mg / ml L. Myeloperoxidase (MPO) 500ng / ml, interleukin-6 (IL-6) 350pg / ml, lipoprotein-associated phospholipase (LP-PLA2) 1000μg / L;
[0055] 2) The contents of the inflammatory markers in the medium-level inflammation composite quality control product are procalcitonin (PCT) 1ng / ml, C-reactive protein (CRP) 5mg / L, serum amyloid A (SAA) 20mg / ml L. Myeloperoxidase (MPO) 400ng / ml, interleukin-6 (IL-6) 150pg / ml, lipoprotein-associated phospholipase (LP-PLA2) 400μg / L;
[0056]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com