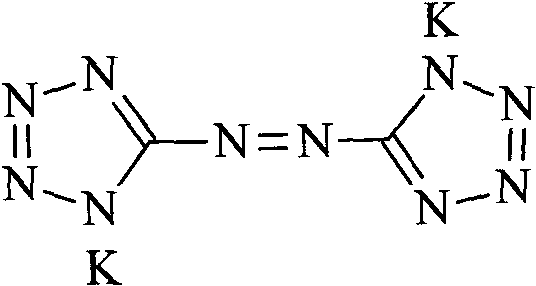
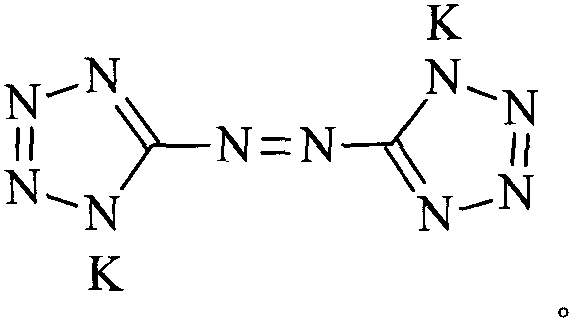
Potassium azotetrazole and its preparation method and use
A kind of technology of potassium azotetrazole and compound, which is applied in the field of energy-containing flame suppressant—potassium azotetrazolium and its preparation, which can solve the problems of lower combustion temperature, high hygroscopicity, low energy, etc., and achieve good flame suppression Effect, reduced flame area, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Potassium hydroxide (224g, 4mol) and 2l of water were added to a 3l four-necked flask equipped with a thermometer, a condensing reflux device and a stirrer, then 5-aminotetrazole (100g, 1.2mol) was added, and heated to a temperature of 60°C When , potassium permanganate (190 g, 1.2 mol) was added, and after the addition was completed, the reaction was carried out for 20 minutes. Add absolute ethanol to reduce excess potassium permanganate, raise the temperature to 95°C, and react for 30 minutes. Filtrate hot, wash the filter cake with hot water, combine the filtrate and washings, concentrate under reduced pressure to a volume of about 1 l, freeze to precipitate crystals, and filter to obtain 140.2 g of an orange-yellow crystalline solid, with a yield of 78.9%.
[0019] Structural identification: IR, ν max (cm -1 ): 3396(N-H), 1652(C=N), 1615(-N=N), 1391, 1189, 1155, 1039(C-N), 729 (tetrazole ring skeleton vibration).
[0020] Elemental Analysis C 2 N 10 K 2 ·3H 2...
Embodiment 2
[0024] Potassium hydroxide (280g, 5mol) and 2l of water were added to a 3l four-neck flask equipped with a thermometer, a condensing reflux device and a stirrer, then 5-aminotetrazole (100g, 1.2mol) was added, and heated to a temperature of 60°C , add potassium permanganate (95 g, 0.6 mol), and react for 20 minutes after the addition is complete. Add absolute ethanol to reduce excess potassium permanganate, raise the temperature to 95°C, and react for 30 minutes. Filtrate hot, wash the filter cake with hot water, combine the filtrate and washings, concentrate under reduced pressure to a volume of about 1 l, freeze to precipitate crystals, and filter to obtain 74.8 g of an orange-yellow crystalline solid, with a yield of 42.1%.
Embodiment 3
[0026] Potassium hydroxide (336g, 6mol) and 2l of water were added to a 3l four-neck flask equipped with a thermometer, condensing reflux device and stirrer, then 5-aminotetrazole (100g, 1.2mol) was added and heated to a temperature of 60°C When , potassium permanganate (316g, 2mol) was added, and after the addition was complete, the reaction was carried out for 20 minutes. Add absolute ethanol to reduce excess potassium permanganate, raise the temperature to 95°C, and react for 30 minutes. Filtrate hot, wash the filter cake with hot water, combine the filtrate and washings, concentrate under reduced pressure to a volume of about 1 l, freeze to precipitate crystals, and filter to obtain 115.6 g of an orange-yellow crystalline solid, with a yield of 56.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com